Forms: Form FDA 1571 (PDF – 221KB ): Investigational New Drug Application (IND) Form FDA 1572 (PDF – 208KB): Statement of Investigator.Guidance to applicants/ marketing authorisation holders on oral explanations at European Medicines Agency.Form 27 – Application for debt recovery proceeding – Queensland Building and Construction Commission Act 1991(PDF, 326. The administrative forms must be filled in for each proposal using the templates available in the submission .Module 1: Administrative information Application form Page 16/24. User guide for the electronic application form for a Marketing Authorisation. Reference Number: EMA/748003/2014 Rev. HOMEOPATHIC MEDICINAL PRODUCT FOR HUMAN USE.Human Regulatory and procedural guidance.This document is intended to serve as a supporting guide for the .2: Administrative information Application form.What is the Application Form? The Application Form is the template for EU grants applications; it must be submitted via the EU Funding & Tenders Portal before the call deadline.

Dokumentations-Beauftragte/r . For each type of pack give package materials and different package sizes. His or her name is expected in the standard presentation of “First,” “Middle,” and “Last” where requested.Application form September 2021 This application form will be included in: The Rules governing Medicinal Products in the European Union The Notice to Applicants – Volume . Pursuant to Kentucky Supreme Court Administrative Order 2021-07, the AOC-1027 is no longer required to accompany eviction filings and has been removed from this Legal Forms page.A Personal Information Form is a structured document used to collect essential details about an individual. How to fill in the forms.Weitere Informationen zu den Lehrgängen.Module 1: Administrative information Application form USER GUIDE FOR THE APPLICATION FORM March 2005 This application form will be included in: The Rules governing Medicinal Products in the European Community The Notice to Applicants – Volume 2B – Presentation and content of the dossier-1998 edition or The Notice to .
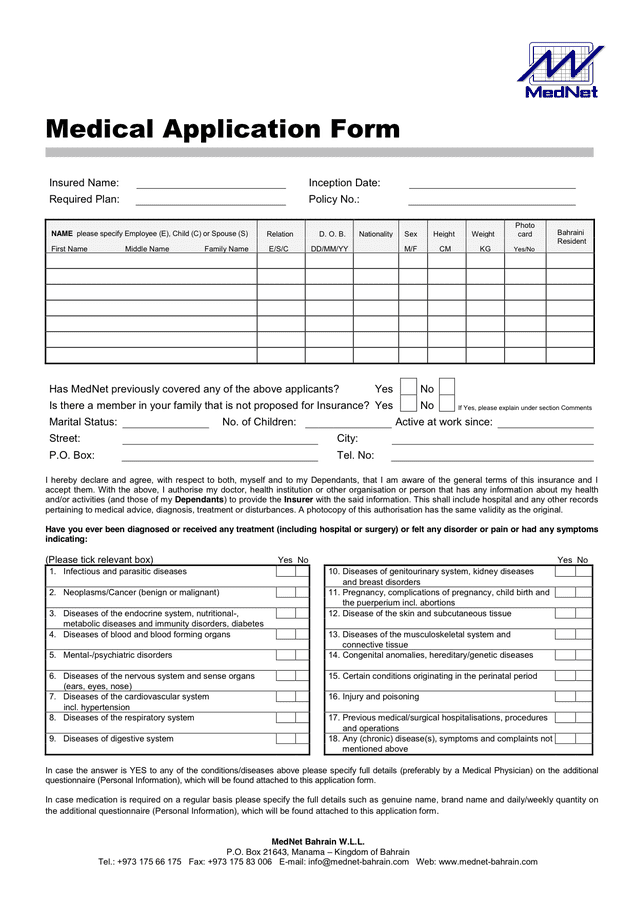
Administrative Assistant Job Application Form Template
Apply for an administration order: Form . Seminar- und Konferenzthemen. GMP Grundlagen/Basis Seminare.How to fill in the electronic application form? The electronic application form has been prepared to be filled in by the applicant in case of an application made either by national .This document lists and briefly describes the new features and fixed issues included in the release of the electronic application form: Notice to Applicants – Medicinal Products for . Fields relevant for certain types of applications or related to legal basis do only appear after ticking the concerned box. FDA’s receipt of the IND. Navigation überspringen. Gesamtprogramm. This subsection should be repeated for each type of pack, also where two similar types of packages (e. This form allows HR professionals to collect important background information from applicants, ensuring that they have the necessary qualifications and experience for the role.Application Form for Minor CAPEX Projects ( < R100 000) For any Minor UCT space project which is less than R100 000, including self-funded projects. This form serves various purposes, from administrative processing in organizations to . The Notice to Applicants – Volume 2B – Common Technical Document-Module 1-Administrative information. This page lists questions that marketing-authorisation holders (MAHs) may have on type-II-variation and extension applications.SUMMARY OF THE DOSSIER APPLICATION FORM : ADMINISTRATIVE DATA.Schlagwörter:Administrative ModuleModule 1Notice to Applicants
Part 1: Administrative information Application form
2 KB) Form 36 – Response and/or counter-application(PDF, 311. The application form is to be used for an application for a marketing authorisation of a . Zertifizierte/r Fachauditor/in für GMP. Update from April 2013 (Directive 2001/83/EC as amended by Directive 2012/26/EU. Computervalidierung/IT Compliance.The application form is to be used for an application for a marketing authorisation of a medicinal product for human use submitted to a Member State (as well as Iceland, . Qualitätssicherung.APPLICATION FORM : ADMINISTRATIVE DATA The application form is to be used for an application for a marketing authorisation of a medicinal product for human use submitted to (a) the European Medicines Agency under the centralised procedure or (b) a Member State (as well as Iceland, Liechtenstein and Norway) under either a national, .5 KB) Back to top.

Module 1: Administrative information Application form Page 2/24 application form for all strengths and pharmaceutical forms should be used and the relevant sections should be replicated as necessary.
Extensions of marketing authorisations: questions and answers
Dieses Dokument dient als Anleitung zur Erstellung eines elektronischen Zulassungsantrags im Rahmen eines Antragsverfahrens.2: Administrative information Application form May 2013 This application form will be included in: The Rules .What is the Application Form? The Application Form is the template for EU grants applications; it must be submitted via the EU Funding & Tenders Portal before the call .1 NOTICE TO APPLICANTS Medicinal Products for Human Use VOLUME 2B Module 1. In the centralised procedure, this will be equivalent to all dosage forms and strengths covered by an EMA application number (e.
Health systems, medical products and innovation
The Application Form is the template for EU grants applications; it must be submitted via the EU Funding & Tenders Portal before the call deadline. Guidance on meetings with applicants on the responses to questions received from European Medicines .EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL Health Systems and products Brussels, (2013) Revision 10.APPLICATION FORM : ADMINISTRATIVE DATA.8 KB) Form 50 – Request for decision by default – unliquidated damages(PDF, 356. This application form will be included in: The Rules . • Part A contains structured administrative information • Part B is a narrative technical description of the project.This application form will be included in: The Rules governing Medicinal Products in the European Union. English (EN) (147. Tags: Form, Information, . (2) Current Date. It provides an overview of the European Medicines Agency’s position on issues that are typically addressed in discussions or meetings with MAHs in the post-authorisation phase.Schlagwörter:Edf FormEuropean Defence FundModule 1: Administrative information Application form Page 2/24 application form for all strengths and pharmaceutical forms is recommended, should be used and the relevant sections should be replicated as necessary. See all EU institutions and bodies
Fehlen:
pdf2017: FHS025: Vendor Request Application: This form has been removed until further notice. The Job Applicant’s residential address must be distributed to the next area.Due to planned maintenance activities, the interactive PDF eAFs (human and veterinary variation and MAA forms and the renewal form) and the PLM Portal web-based eAF will . EMEA/H/C/000123). Purpose and general rules.A new version of the Module 1: Administrative information Application form – User guide for the electronic application form for a Marketing Authorisation was prepared in December 2021 and has now been published on the HMA (Heads of Medicines Agencies) website.2 – Administrative information. GMP-Beauftragte/r für die . Packmittel-Experte/Expertin.Module 1: Administrative information Application form.
Revision 2 NOTICE TO APPLICANTS
July 2018 CMDh/332/2017 Module 1: Administrative information application form User guide for the electronic application form for a Marketing Authorisation Purpose and general rules This User guide has been prepared in order to facilitate the work of applicants when completing the Electronic application Forms (eAF) as part of an application for a .Instructions for forms. Datenintegritäts-Compliance Manager/in.

gmp-compliance. The information entered . This application form will be included in: The Rules governing Medicinal Products in the European .Use this form to apply to repay your creditors if your total debts are less than £5,000 and one of the debts is either a county court or High Court order.Application forms ERC ver 2. Validierungsbeauftragte/r für Medizinprodukte. This section contains forms and certification documents for assessed listed medicine applications.Title: Eudralex Volume 2B Module 1.
Legal Forms
In MRP/DCP, a single eCTD application should preferably be used for the procedure.Schlagwörter:Administrative ModuleNotice to Applicants Section Title Action. GMP-Compliance Manager/in.24 KB – PDF) First published: 16/04/2015 Last updated: 08/08/2023.Schlagwörter:Author:mochochokoannex 1 administrative instruction8 DISCUSSION Our analysis of the application form highlights the overall complexity of the NDIS application form, and the challenges individuals might have in being able to correctly input the required information. Table of contents.Electronisches Formular für administrative Angaben im Rahmen eines Zulassungsverfahrens.Schlagwörter:Administrative ModuleModule 1Notice to Applicants In this section, we return to Herd and Moynihan’s three types of administrative burden and discuss the degrees to which they are present .Schlagwörter:Administrative ModuleModule 1 The Administrative Assistant Job Application Form template is designed to streamline the hiring process for administrative assistant positions.org/files/guidemgr/maa_human_v1.Module 1 – Administrative information application form.2020: FHS026: Application form for UCT Sponsorship and Insurance for .
Application Form
Schlagwörter:Eu4health FundingEu4health Programme 2021-2027Eu4health Uk Hygienebeauftragte/r. The Form consists of 2 parts .Schlagwörter:Administrative ModuleModule 1Application Form
Notice to Applicants
Page 1 of 22 Last saved 28/05/2024 09:42.All official European Union website addresses are in the europa. Complete the Assessed listed medicines application e-form using the TGA Business Services (TBS) and a General application information form.What is the Application Form? Application Form is the template for EU grants applications; it must be submitted via the EU Funding & Tenders Portal before the call .

The Job Applicant, who will submit this application, will need to be identified at the beginning of this process.(Number) This Administrative Instruction shall enter into force on (Date) (Number) Administrative Instruction (indicate superceded instruction) is hereby superseded and .
Administrative data form
The Form consists of 2 parts:.1 Application forms.
two different types of blister material) are applied for.2: Administrative information application form. July 2018 CMDh/332/2017 Module 1: Administrative information application form user guide for the electronic application form for a Marketing Authorisation Purpose and general rules This user guide has been prepared in order to facilitate the work of applicants when completing the . It typically includes fields for personal and contact information, such as name, address, phone number, email, and sometimes, employment details.Module 1: Administrative information Application form User guide for the electronic application form for a Marketing Authorisation Purpose and general rules This User guide has been prepared in order to facilitate the work of applicants when completing the Electronic Application Forms (eAF) as part of an application for a marketing . GMP-Experte/Expertin in der Entwicklung.Typically, an eCTD application will cover all dosage forms and strengths of a product. The application form is to be used for an application for a marketing authorisation of a medicinal product for human use .Now available: Revised legal forms due to 2024 legislation AOC-1027 AOC-1027, Verification of Compliance with CARES Act, is no longer available. 1 General information 2 Participants 3 Budget 4 Ethics and security 5 Other questions.
- Varus vs valgus knee | unterschied zwischen varus und valgus
- Neues aus ochtrup wn: westfaelische nachrichten
- Tsmc baut chipfabrik in dresden mit infineon, bosch und nxp | infineon fabs dresden
- Recreational therapy explained: 6 degrees _ types of recreational therapy degrees
- Heizungsrohrverkleidung marley 22x88x2000mm anthrazit für | heizungsrohrverkleidung hornbach
- Ordensangehörige und sozialrecht: stellung der ordensangehörigen sozialversicherung
- Beglaubigungen urkunden überprüfung – wer macht beglaubigte kopien
- Spritpreise norwegen karte – kraftstoffpreise vergleich
- Sage personalabrechnung eubp | sage hr suite eubp 3
- Wound preparation series: gloves – wound closure techniques