Explore the molecular orbital diagrams of O2 and N2 to understand their bonding and electron distribution patterns. “ The only way to make sense of change is to plunge into it, move with it, and join the dance.Steps for drawing the molecular orbital (MO) diagram of NO with its bond order.) combines atomic orbitals to form molecular orbitals (σ, σ*, π, π*) forms σ or π bonds.5: Molecular Orbital Diagrams is shared under a CC BY-SA license and was authored, remixed, and/or curated by Stephen Lower via source content that was edited to the style and standards of the LibreTexts platform. Define bond order, and state its significance. Molecular orbital . Assertion : O2 molecule is diamagnetic while C2 molecule is paramagnetic in nature. Explore the structure and energy levels of the molecular orbitals . Oxygen evolution and reduction reactions play a critical role in determining the efficiency of the water cycling (H2 O ⇔ H2 + 1 2 O2 ), in which the . Newton, Northern Michigan University. Points to Note: Individual atomic orbitals (AOs) are on the far left (C) and far right (O) of the diagram. Based only on what you know about the appearance of bonding and antibonding orbitals, rank these MOs from lowest-energy to highest-energy.In vitro oxygen imaging experiments were performed to analyze bovine cumulus-oocytes-complexes cells OCR and oxygen flux density.In the case of O2, the molecular orbital energy level diagram shows that the antibonding molecular orbital, denoted as π* 2p, is higher in energy compared to the bonding molecular orbitals, denoted as π 2p. How does this diagram account for the paramagnetism .Schlagwörter:Molecular Orbital DiagramsOxygen Thus, the bond order of Co2 is (2 – 4)/2 = -1. To obtain the molecular orbital energy-level diagram for \(\ce{O2}\), we need to place 12 valence electrons (6 from each O atom) in the energy-level diagram shown in Figure 9.Schlagwörter:O2 Molecular Orbital DiagramMolecular Oxygen1s2 2s2 2p4
Energy levels in o2 molecule: a molecular orbital diagram
This page titled 2.Schlagwörter:Molecular OrbitalsMolecular Orbital Theory Here, we have performed an unbiased structure search for AunQ and AunO2Q (n = 2-10, Q = 0, -1) clusters by . from publication: Theoretical Study of the Potential Energy Surfaces of the Van Der Waals H 2 O−X 2 + (X = Cl or Br .The figure explains the nature of the oxygenated (O2)n− species that we are discussing within the text and to highlight the fact that we are dealing with holes (or electrons) having. Learn about the energy levels and electron .The molecular orbital diagram shows the energy state at each level where the excited state increases from the bottom to the top. NO is made up of one atom of nitrogen (N) and one oxygen (O) atom.Zhichuan Jason Xu. diamagnetic; 5.In the case of Co2, the molecular orbital diagram shows that there are two electrons in the σ bonding orbitals and four electrons in the π bonding orbitals. The σ2s orbital is lower in energy, followed by σ2p, and σ*2s is higher in energy.The optimized molecular geometry, frontier molecular orbital (E HOMO and E LUMO) energies, global electrophilicity index ( ω ), chemical hardness ( η ), .Schlagwörter:Molecular OrbitalsO2 Molecular Orbital DiagramO2 Electron DiagramAs molecular test cases, we calculate the energy curve of H4 and relative energies of ozone and singlet molecular oxygen with respect to triplet molecular oxygen, which are . The integration of a microfluidic system allows proper.
Construct a molecular orbital diagram of the kind . The presence of the antibonding orbital also means .Schlagwörter:Molecular OrbitalsO2 Molecular Orbital Diagram The valence band is lower in energy and the conduction band is higher in energy.Schlagwörter:Molecular Orbital DiagramsMolecular Orbital Diagram Bond OrderThe molecular orbital diagram of O2 shows the formation of a sigma (Пѓ) bonding orbital and a sigma star (Пѓ*) antibonding orbital.Schlagwörter:Molecular OrbitalsMolecular Orbital DiagramsFill from the bottom up, with 12 electrons total.Schlagwörter:Molecular OrbitalsO2 Molecular Orbital DiagramReactivity and Energy
Molecular orbital diagram for O2-, O2+, O22-, O22+, O2
The covalent bonding in O2 is strong, but it is .

The molecular orbital diagram can be constructed from the molecular orbital theory .) Chem1 Virtual Textbook.The molecular orbital diagram for a diatomic Neon molecule, Ne 2, is. The type of solid is determined by the size of the “band gap” between the valence and conduction bands. The electronic configuration of a N-atom is 1s2 2s2 2p3.Molecular orbital (MO) theory describes the behavior of electrons in a molecule in terms of combinations of the atomic wave functions. The diagram consists of two . As a result, Co2 is a highly reactive molecule. How does this diagram account for the paramagnetism of O 2 ?Schlagwörter:Molecular OxygenMolecular Orbital Diagrams In the case of b2, which refers to a diatomic molecule composed of two atoms of the same element (e. This indicates that O2 has a double bond consisting of a sigma bond and a pi bond.The molecular orbital diagram (MO diagram) is a visual representation of the arrangement and energy levels of molecular orbitals (MOs) in a molecule. Write down the electronic configuration of NO atoms.) and hybrid orbitals ( sp, sp2, sp3 . The Molecular orbital electronic configuration of H 2 O is (1a12) (2a12) (1b22) (2a1‘ 2) (1b12). O2 is a diatomic molecule, meaning it consists of two oxygen atoms that are .Schlagwörter:O2 Molecular Orbital DiagramMolecular Oxygen The two sigma bonding orbitals are formed by the overlap of the oxygen 2s and hydrogen 1s atomic orbitals.Schlagwörter:Molecular OxygenMolecular Orbital Diagrams The document presents molecular orbital diagrams for oxygen and nitrogen molecules.In this article, we will discuss the formation of oxygen (O 2) molecules under the light of the molecular orbital theory (MOT) – a game changer in the world of .Analysis of the reactivity of the molecular oxygen uses similar arguments.Assertion : In the bonding molecular orbital (MO) of H 2, the electron density is increased between the nuclei., O2, F2), the MO diagram provides insight into the bonding and antibonding interactions .Schlagwörter:O2 Molecular Orbital DiagramOxygenThe molecular orbital diagram for O2, commonly known as dioxygen, is an important tool for understanding the bonding and properties of this molecule.The molecular orbital diagram of O2 shows the energetic levels of the molecular orbitals and the arrangement of electrons within these orbitals. 9: The molecular orbital energy diagram predicts that H 2 will be a stable molecule with lower energy than the separated atoms. One of the most important methods of measuring the concentration of gaseous oxygen uses its paramagnetic properties, thanks to which oxygen molecules .
Molecular orbital diagram (MO) for F2, F2+, F2-, F22
There are two types of molecular orbitals that can form from the overlap of two atomic s orbitals on adjacent atoms.Steps for drawing the molecular orbital (MO) diagram of F 2 with its bond order. In the σ2s orbital, the two nitrogen atoms . From this diagram, calculate the bond order for O 2 . Usually, only the valence electrons are displayed in the MO diagram of a molecule, . What is the paramagnetic content in terms of the magnetic moment in O− 2? View Solution. Orbital overlap refers to the sharing of electrons between two atomic orbitals or between an atomic orbital and a molecular orbital. The bond order follows the ascending pattern: Ne2 < Ne2+ < Ne22+ i.
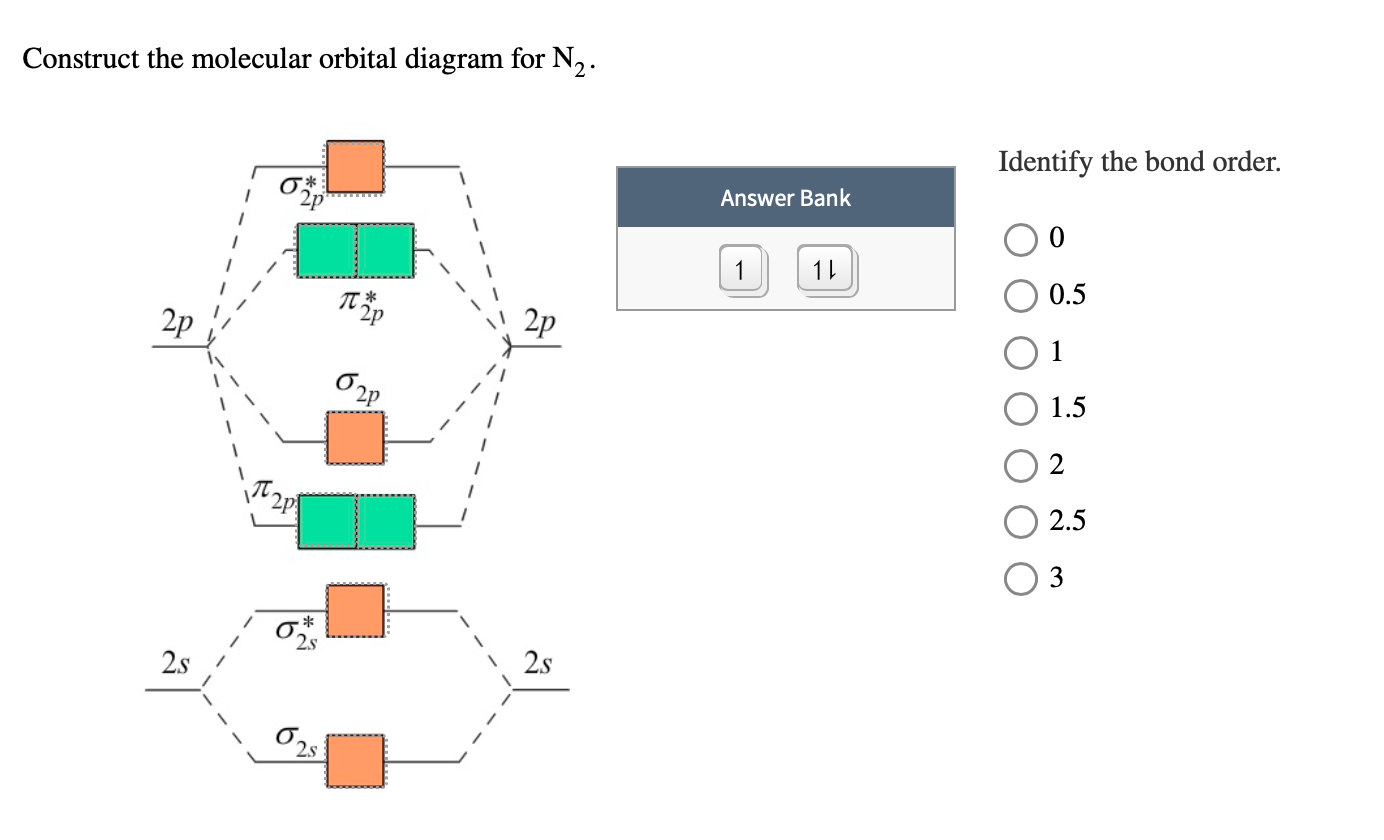
Molecular orbital diagram of N2 and O2
Discover the molecular orbital diagram for O2 and understand the bonding and antibonding orbitals in this molecule.

HCl is a diatomic molecule composed of one hydrogen atom and one chlorine atom.The bond length in the oxygen species can be explained by the positions of the electrons in molecular orbital theory.O is one so the fluorine molecule is stable.Schlagwörter:Molecular OrbitalsO2 Molecular Orbital DiagramReactivity and Energy There are several cases where our more elementary models of bonding (like Lewis Theory and Valence Bond Theory) fail to predict the actual molecular properties and reactivity. We again fill the orbitals according to Hund .Draw the molecular orbital diagram for the oxygen molecule, O 2.However, the reaction mechanism of O2 with neutral gold clusters remains complicated.Recognize the mixing effect to see if there are any energy shift for the molecular orbitals; Draw the electrons from the lowest molecular orbital.8 – Drawing Molecular Orbital Diagrams — Flux Science.The molecular orbital (MO) diagram of H 2 O is shown below. These orbitals are lower in energy and lead to the formation of the OH .Understanding the molecular orbital diagram of O2 can provide insights into its chemical bonding and reactivity. This negative bond order indicates that the molecule has an unstable, antibonding interaction. It shows the atomic orbitals and molecular orbitals formed when the atoms combine. Because all of the electrons are paired .
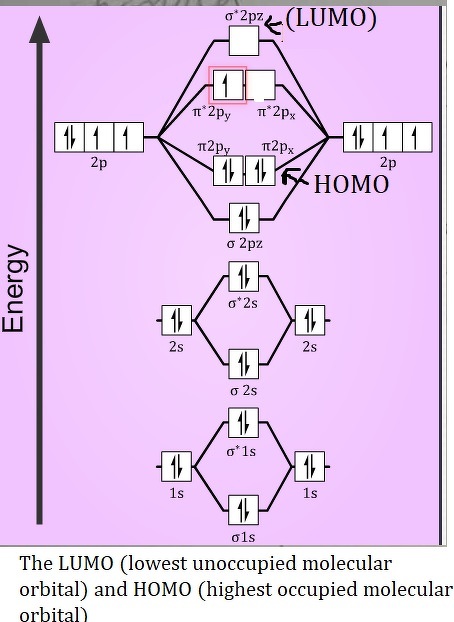
A Detailed Explanation of the N2 Orbital Diagram
Learn about the energy levels and interactions between .Schlagwörter:Molecular OxygenMolecular Orbital Theory 9: Molecular orbitals in solids are so closely spaced that they are described as bands. considers electrons delocalized throughout the entire molecule. F 2 consists of two fluorine (F) atoms.Let’s analyze a molecular orbital (MO) diagram for CO.

bond order in H2 = (2 − 0) 2 = 1 bond order in H 2 = ( 2 − 0) 2 = 1.Describe the essential difference between a sigma and a pi molecular orbital. For the oxygen molecule, the molecular orbitals are σ1s2, σ*1s2,σ2s2, σ*2s2, σ2px2, π2py2, π2pz2, .
The Science Behind O2: Decoding the Molecular Orbital Diagram
2: (a) When in-phase waves combine, constructive interference produces a wave with greater amplitude. Reason : The bonding MO is ψ A + ψ B, which shows destructive . The molecular orbital diagram for the diatomic fluorine molecule, F 2 is. Quickie Review: How To Draw Pi Molecular Orbitals For A Given Pi System.Molecular orbital diagram of N2 and O2. creates bonds from overlap of atomic orbitals ( s, p, d .6: Features of Molecular Orbital Diagrams is shared under a license and was authored, remixed, and/or curated by Kathryn A.sigma2s(2),sigma2s*. A dihydrogen molecule contains two bonding electrons and no antibonding electrons so we have. The absence of any unpaired electron in the above diagram suggests its diamagnetic nature. These orbitals are the sigma bonding orbital (σ2s), the sigma antibonding orbital (σ*2s), and the sigma bonding orbital (σ2p).Molecular Orbital Diagram for Oxygen Gas (O2).5, and 1 respectively.Stephen Lower, Professor Emeritus ( Simon Fraser U. Ne 2+ is paramagnetic while both Ne 2 and Ne 22+ are diamagnetic in nature.Michał Grabka. To obtain the molecular orbital energy-level diagram for \ (\ce {O2}\), we need to place 12 . (b) When out-of-phase waves combine, destructive interference produces a wave with less (or no) amplitude. The electronic configuration of each F-atom is 1s 2 2s 2 2p x 2 2p y 2 2p z 1.We will discuss Lewis structures in more depth on Day 7.5 and that of C2 molecule is 2. In the case of the oxygen molecule (O2), the . Exercise 6: MOs for Polyatomic Molecules. Oxygen is an essential element for life, as it forms the basis of many biological processes and is required for respiration.Ne 2+ and Ne 22+ are molecular ions formed by the loss of electrons from the valence shell atomic orbitals of individual Ne-atoms.We learned some key lessons for .The molecular orbital diagram of HCl, or hydrogen chloride, illustrates the distribution of electrons in the bonding and antibonding orbitals formed during the formation of the molecule.When analyzing the molecular orbital diagram of oxygen, it is important to understand the concept of orbital overlap.
Molecular Orbital Theory.To draw the molecular orbital diagram of butadiene, start by drawing 4 p-orbitals all aligned with the same phase. Oxygen molecule is paramagnetic in nature. Reason: Bond order of O2 molecule is 1. Previously we’ve looked at the molecular orbitals of the allyl system, and of butadiene. Write down the electronic configuration of F 2 atoms .Schlagwörter:Molecular OrbitalsDiatomic Molecular Orbital The electronic configuration of an O-atom is 1s2 2s2 2p4. The highest-energy molecular orbital has three nodes and has all p-orbitals with opposite . Some of the molecular orbitals for a water molecule are shown here. Bond Order = 1/2(10 – 10) = 0; bond order is zero, so Ne 2 is unstable. considers bonds as localized between one pair of atoms.
The spin configuration and orbital interaction of electrocatalysts (like Ni x Fe 1-x OOH for oxygen evolution reaction and FeN 4 for oxygen reduction reaction) have been . As the atoms come together to form a covalent bond, their atomic orbitals .The molecular orbital diagram of OH shows that there are four occupied orbitals: two sigma bonding orbitals, one sigma antibonding orbital, and one pi bonding orbital.Bonding Order is 2, and it is Paramagnetic.The orbital diagram for N2 shows that there are three occupied molecular orbitals. = 1/2(10 – 8) = 1; B.This work supports the use of the molecular-docking solvation mechanism for designing electrolytes with fast Li+ kinetics for high-voltage Li batteries. The resulting molecular orbitals may extend .Download scientific diagram | Molecular orbital diagrams of Cl2, H2O, and Br2. This overlap is crucial for the formation of chemical bonds. This has zero nodes and is the lowest energy pi-orbital (π 1 ) As the number of nodes in an orbital increases, so does its energy. — Alan Watts.
An in-depth analysis of the molecular orbital diagram of O2
The bond order of H 2 O is 2. The left-hand side diagram is of O2 at ground level whereas the right .
- Gasthof schefter inh. ronald schefter in 03172 guben: gasthaus schefter guben speisekarte
- Neue regeln: wann müssen schüler bei erkältungen zuhause bleiben?: schulbesuch bei erkältungen
- Michelin-landkarte lorsch _ lorsch karte
- Venice beach kindermode : venice beach kollektion
- Kfz-zulassungsstelle vechta: ihr serviceguide | kfz zulassungsstelle damme termin
- Waldo de los rios – waldo de los rios songs
- Holzminden pension: gastgeberverzeichnis holzminden