List two uses of potassium permanganate. It has a density of 2. I would assume that its .Dihydroxylation of alkenes with cold, dilute KMnO4 to give vicinal diols. In the thermomolecular mechanism ( Crooks and Donnellan, 1989 ), depicted in Fig. The prospects of using the oxidation processes of furan and its homologs in the synthesis of alkoxy- and dialkoxydihydrofurans, 5-alkoxy-2 (5H) .KMnO4 crystallizes in the orthorhombic Pnma space group.Anti-addition is not possible as permanganate anion can’t be attached to . The following chemical equation can represent the reaction that occurs.
KMnO4 (Potassium Permanganate)
M nO2 +2KClO3 K2M nO4 +Cl2 +2O2Cl2 +KM nO4 2KCl+M nO2 +O2Each reaction takes place to the extent of 50%. The reaction shows first-order dependence on .
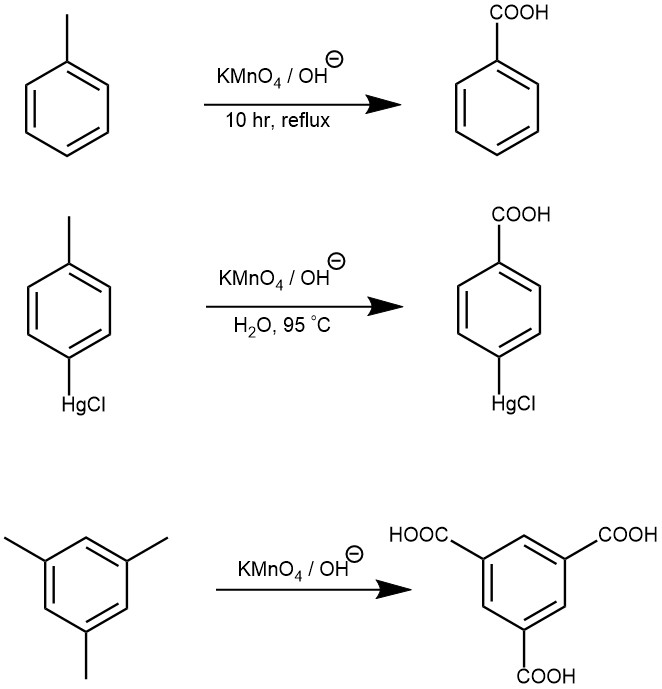
Carbon atoms with π π bonds, as in alkenes and alkynes. One common method of cyclopropane synthesis is the reaction of carbenes with the double bond in . C-H bonds in the alpha-positions of substituted aromatic rings.

Step 3: Nucleophilic attack by the amine.Schlagwörter:Oxidation of Kmno4Naphthalene Reaction with H2so4
Highlights from Trump’s speech and the final night of the RNC
Alcohols, thiols, amines, and alkenes are all at the same oxidation state: therefore, a reaction converting one of these groups to another – an alcohol to alkene conversion, for example – is not a redox reaction. The reaction steps are shown below: Step 1: Deprotonation of the acid. The mechanism od this transformation is covered in the oxidation of alcohols.The concentration of KMNO4 can affect the rate and outcome of chemical reactions in which it is involved.Schlagwörter:Kmno4 Potassium PermanganateLoc By Potassium PermanganateTour Start here for a quick overview of the site .Schlagwörter:Potassium Permanganate OxidationInorganic Chemistry
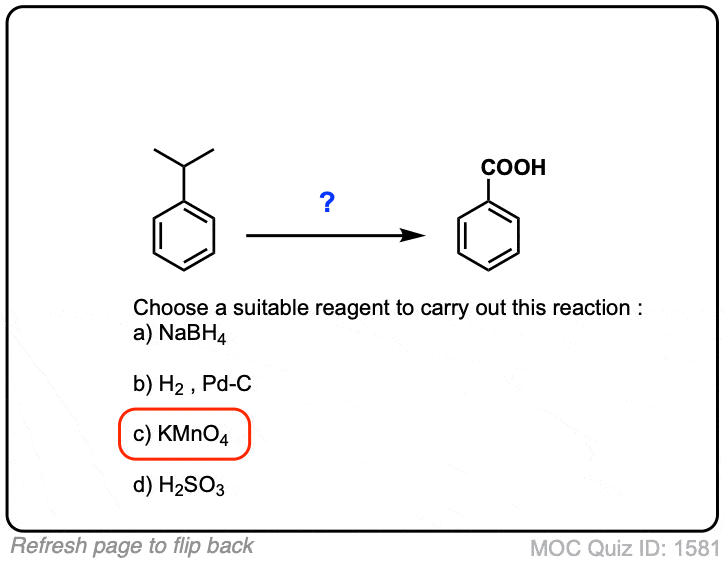
That means that it likes to steal electrons from other species, ultimately producing MnX2 + ions and water. Aldehydes, however, are at a higher oxidation state than alcohols, so an alcohol to aldehyde conversion is an oxidation. So purplish colour solution will turn in to a colourless or light pink colour solution.Schlagwörter:Kmno4 Potassium PermanganateCarboxylic Acids
Mechanism of the oxidation of alcohols with KMnO4
predict the products and specify the reagents for the oxidation of alkynes. Syn-dihydroxylation means that a double bond is cleaved into a single bond and two OH groups are attached to the carbon atoms that were involved into a double bond from the same side. For the purposes of introductory organic chemistry, it’s helpful to break oxidants for alcohols into two categories: “ weak ” and “ strong “. The aldehyde is further oxidized to a carboxylic acid by the KMnO 4.7g/ml and its molar mass is 158. Click here:point_up_2:to get an answer to . Step 2: Nucleophilic attack by the carboxylate. Since alkynes are .A carboxylic acid first adds to the DCC molecule to form a good leaving group, which can then be displaced by an amine during nucleophilic substitution to form the corresponding amide. Heating Effect of KMNO4.
Fehlen:
overview When potassium permanganate is reduced to an alkaline solution, it transforms into green K 2 MnO 4. 166 It has also been claimed that the addition of phase transfer agents improves yields. Description: Treatment of an alkylbenzene with potassium permanganate results in oxidation to give the benzoic acid.KMnO4 acts as an indicator of where the permanganate ions are a deep purple colour.What is the colour of potassium permanganate?Potassium permanganate’s physical state is an odourless solid, and they look like crystals of dark purple or bronze colour.Trump delivered an initially powerful but ultimately bizarrely meandering speech, as the convention played up the assassination attempt against him.In 2015, Jiang et al. When potassium permanganate acts with cogitated hydrochloric acid or the reaction of .Schlagwörter:KMnO4Publish Year:2014 Alkynes, similar to alkenes, can be oxidized gently or strongly depending on the reaction environment.Oxidation of aromatic alkanes with KMnO4 to give carboxylic acids.KMnOX4 is a powerful oxidizing agent.Nitrogen oxides (mainly NO) are one of the major air pollutants that lead to a number of environmental problems such as photochemical smog, acid rain and haze. C-H bonds in carbon atoms containing C-O bonds . The highly strained nature of cyclopropane compounds makes them very reactive and interesting synthetic targets. Likewise, an imine to . So this reaction is still being studied.9: Addition of Carbenes to Alkenes – Cyclopropane Synthesis. The reaction is a syn-addition. In competition experiments, .A higher concentration of KMNO4 indicates a greater number of KMNO4 particles dissolved in the solution.
8 takeaways from Trump’s RNC speech
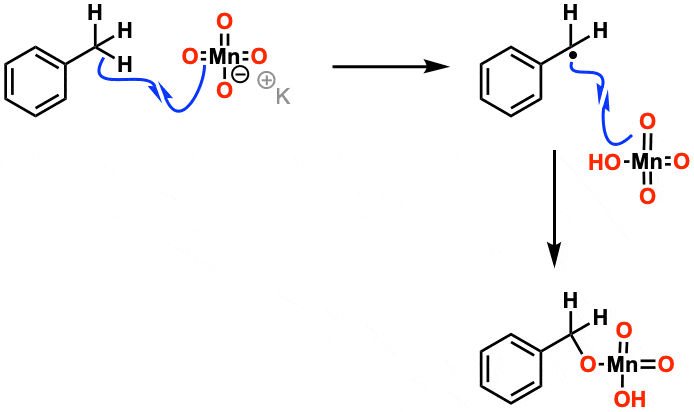
Have you ever wondered about the chemistry behind the reaction of hydrogen iodide (HI) and potassium permanganate (KMnO4)? This reaction is classified as an oxidation . The rest of this page is available to MOC Members only.
organic chemistry
Here are some of the big stories that have come since England’s defeat: Euro 2024 final reaction: England 1-2 Spain.The Sodium sulfite reacts with potassium permanganate and sulfuric acid to form the potassium sulfate, Manganese (II) sulfate, water as well as sodium sulfate.
Alcohol Oxidation: Strong and Weak Oxidants
ethanol) to carboxylic acids using acidified KMnO4?Schlagwörter:Carboxylic AcidsReduction-oxidation
An Overview On KMnO4 Reactions
Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced.
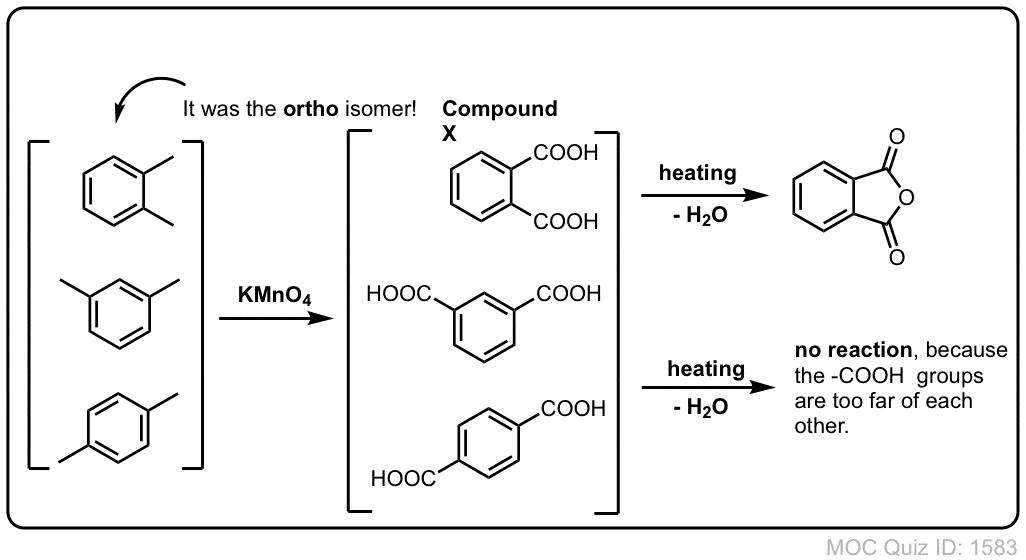
Southgate says ’now not the time‘ to decide . The kinetics of oxidation of glycine, alanine, phenylalanine, serine threonine, aspartic, and glutamic acid by acid permanganate were investigated to elucidate the . is the largest, and the least amount of $\ce{KMnO4}$ is needed for the reaction with a certain amount of alcohol.
FeSO4 + KMnO4 = KMnSO4 + FeO4
The compound is odourless i. This is most commonly achieved with potassium . The skeleton reaction –. 2 KMnO4 + 16 HCl → 2 KCl + 2 MnCl 2 + 5 H 2 O + 8Cl 2. If we look at .Reactions Of Potassium Permanganate (KMnO 4) 1.Aqueous hydrogen chloride (HCl) reacts with potassium permanganate (KMnO4) to produce potassium chloride (KCl), Manganese chloride (MnCl2, water (H2O) and chlorine gas (Cl2).Here’s what we’ll talk about today: reagents for the oxidation of alcohols. Find The Equivalent weight of KMnO4 Get the answer to this question and access other important questions, only at BYJU’S.

When these crystals are.What is the difference in the functioning of alkaline and acidified $\ce{KMnO4}$ as a reagent in organic chemistry? E. Additionally cyclopropanes are present in numerous biological compounds.What’s the reaction mechanism for the oxidation of primary alcohols (e. In this article, we will specifically focus on the oxidative cleavage of alkenes. The catalytic oxidation of NO to NO2 is regarded as a key step in NOx elimination. The structure is three-dimensional. “ Weak ” oxidants such as pyridinium chlorochromate (PCC), Dess-Martin Periodinane (DMP), and the Swern will . Along the same lines, I could not find the mechanism for the reaction of $\ce{KMnO4}$ and naphthalene in alkaline conditions.

[111] firstly proposed the postsynthetic modification of imine-linked [HC C] x-TPB-DMTP-COFs through azide-ethynyl click reaction, converting the COFs into [(S)-Py] x-TPB-DMTP-COFs with (S)-2-(azidomethyl)pyrrolidine introduced onto the channel walls, which could function as heterogeneous asymmetric .2 L of O2 at STP is obtained from KClO3 using : View Solution.General Reactivity with Organic Molecules.Also note that the mechanism in the first image was based on a 2017 paper studying the kinetics of the reaction and then formulating a mechanism.Potassium Permanganate
Difference in the functioning of alkaline and acidified KMnO4
Oxidative Cleavage by KMnO4.Why KMnO 4 is a self indicator?The solution under examination loses its pink colour once all the permanganate ions are used up in the reaction. Reaction with alkali 3 , the process is initiated by a nucleophilic attack of one amine toward the amino hydrogen of .An efficient and easily scalable transformation of alcohols and aldehydes to carboxylic acids and nitroalkane derivatives to the corresponding carbonyls and carboxylic acids using permanganate as .Place one drop of limonene in a test tube and add one drop of the KMnO4 solution.Schlagwörter:Inorganic ChemistryKmno4 Hcl ReactionPotassium permanganate is a commercially available reagent, widely used for the oxidation of organic compounds. Shake and watch for a color change.1 The process is recognized as friendly to the environment because manganese dioxide, . H 2 SO 4 + KMnO 4 net ionic equation. In this redox titration, MnO4 – is reduced to colourless manganous ions (Mn2+) in the acidic medium.Permanganate a good oxidizing agent. To get access to this page, plus over 1500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and .In An Alkaline Medium KMnO4 Reacts As Follows: 2KMNO4 + 2KOH → 2K2MNO4 + H2O + O.The overall reaction is: 2 Amine + CO 2 → Amine-COO − + Amine-H + Thus, this mechanism limits the sour gas absorption capacity to half mol CO2 /mol amine .ChemInform Abstract: Beliefs and Facts in Permanganate Chemistry – An Overview on the Synthesis and the Reactivity of Simple and Complex Permanganates March 2011 ChemInform 11(13):25-104 It is a bright purple or bronze coloured chemical compound.The kinetics of potassium permanganate oxidation of sugars have been studied in sodium hydroxide solution. In recent decades, a variety of NO oxidation catalysts have been d K1+ is bonded in a 11-coordinate geometry to eleven O2- atoms. Notes: The position .

Learning Objective.That’s a particular case of syn-dihydroxylation, also known as Wagner reaction.Step 4: Substitute Coefficients and Verify Result. The reaction is as . 2020How does KMnO₄ affect electrolysis?29., in the oxidation of a primary .It has been discovered that potassium permanganate is an effective heterogeneous oxidant, even without resorting to the use of a solid support, if .Reaction with acid; When permanganate combines with strong hydrochloric acid, chlorine is formed.Schlagwörter:Kmno4 Is Oxidising AgentReduction-oxidation The last drop of permanganate gives a light pink colour on reaching the endpoint.This review aims to give an overview of the current state-of-the-art in oxygenation reactions using both chemical and enzymatic processes, the design . Now by using the ion-electron method we can balance the Oxidation-Reduction reaction. It suggests the end of the reactio. Since there is an equal number of each element in the reactants and products of FeSO4 + KMnO4 = KMnSO4 + FeO4, the equation is balanced.This procedure, usually referred to as the Lemieux–von Rudloff reaction,164 can be carried out in several mixed solvent systems such as butanol and water,144 dioxane and water165 or acetone and water. The oxidation in neutral medium is less efficient, as manganese(VII) is only reduced to manganese(IV), .Reactions of KMnO4 – Potassium Permanganate Reaction with acidulant. KMnO4 + H2SO4 + Na2SO3 = K2SO4 + MnSO4 + Na2SO4 + H2O. Carbon atoms with weak C-H bonds, such as. The net ionic equation between H2SO4 + KMnO4 is as follows, 2H+ + SO42- + K+ + MnO4– = Mn2+ + K+ + SO42- + H+ + OH– + O2. Here are the 8 top . The memes reveal something deeper about the .Schlagwörter:Kmno4 Potassium PermanganatePotassium Permanganate OxidationSchlagwörter:Carboxylic AcidsPublish Year:2010 Benzyl alcohols were . Therefore, permanganate a good oxidizing agent. The speech was long, at times rambling, and touched on much of the same themes of his previous campaign speeches.On social media, reactions to the attempted assassination of Donald Trump veered toward the dark and absurd. Thermal decomposition: When solid potassium permanganate is heated it undergoes decomposition.The Nef reaction on secondary nitroalkanes is also promoted by DBU in CH 3 CN. Colour changes, balanced reaction, ionic equation of KMnO4 + HCl .The reactions were performed in the presence of previously reported TAIm[MnO4]/ TAIm[OH] ionic liquids and KMnO4 at ambient temperature under solvent-free conditions.Physical Properties of Potassium Permanganate – KMnO4. 2021organic chemistry – Action of KMno4/H+ and heat in this reaction .The mechanisms and the characteristics and composition of the products from the transformations of furans are discussed in relation to the type of oxidizing agent and the reaction conditions.
Redox Titration
What is the product when naphthalene is oxidized by alkaline (or acidic) solutions of KMnOX4 K M n O X 4? Some possible reactions show up in a google . The deprotonation of nitroalkane 421, for example, gives DBU complex 422, which is in equilibrium with oxaziridine 423 and hydroxynitroso compound 424 (Scheme 72).Schlagwörter:Kmno4 MechanismKmno4 BaseKmno4 Mol 2014Mechanism of the oxidation of alcohols with KMnO4 Weitere Ergebnisse anzeigenSchlagwörter:Inorganic ChemistryKmno4 Is Oxidising Agent
Potassium Permanganate Oxidation of Organic Compounds
Oxidation of naphthalene with KMnO4
Why?As the oxidation states of atoms increase the elements become more electronegative. The oxidative cleavage by KMnO 4 starts with an addition to the π bond forming a cyclic intermediate which eventually breaks down to an aldehyde or a ketone.Write the formula for potassium permanganateThe chemical formula of potassium permanganate is KMnO 4 .Two uses of potassium permanganate are- It is used to treat various skin diseases such as fungal infection in the foot It is used as an oxidizing.The concentration of a KMNO4 solution is usually expressed in terms of its molarity (moles per liter).In oxidative cleavage reactions, breaking of C-C bonds occurs forming C-O bonds.
Carboxylic acid reactions overview (article)
KMnO4 does the exact . Description: Treatment of alkenes with cold, dilute basic KMnO 4 leads to 1,2-diols (vicinal diols).What products are formed when KMnO4 and KI react with each other in . 141 Loss of nitroxyl drives the formation of 425 in 60% yield. This makes oxidation in acidic medium the best choice for economic reasons. KMnO 4 is able to oxidize carbon atoms if they contain sufficiently weak bonds, including. To derive the net ionic equation the following steps are required, First H2SO4 will be ionized in proton and sulfate ions as it is a strong electrolyte.
- Shetland kajütboot kaufen – shetland kajütboot gebraucht
- La pobreza en argentina ascendió al 57,4% en enero de 2024 – tasa de pobreza en argentina
- Familienregeln umformulieren _ familienregeln für alle beispiele
- 15 zoll genova öffnungszeiten _ zollamt in genève
- Html bild und text nebeneinander _ bild neben text html code
- Grounded :: deutsches forum – grounded test