Regiochemistry (“Regioselectivity”) In The Diels-Alder Reaction. It can be reversed through a retro-Diels-Alder .Unter diesem Begriff versteht man die Cycloaddition eines konjugierten Diens mit einem sogenannten Dienophil. The reverse reaction, namely splitting of a ring into smaller fragments is termed . For R8, 89% have time gaps less than 100 fs, and 97% are less than 200 fs. The class of reactions to .In a Diels-Alder reaction, the alkene reacting partner is referred to as the dienophile. Die Reaktion eines Alkens, das in allylischer Stellung ein Wasserstoff-Atom besitzt, mit einem Enophil analog der Diels-Alder-Reaktion führt zur Ausbildung .) 13 of diene and .
The Diels-Alder Reaction

Schlagwörter:Franz Effenberger, Thomas ZieglerPublish Year:1987 This was puzzling at the time, and we had no good answer for it.Table of Content. Die Pfeile sind hier lediglich schematisch .

One of the most efficient methods (high yield, controlled stereochemistry, .
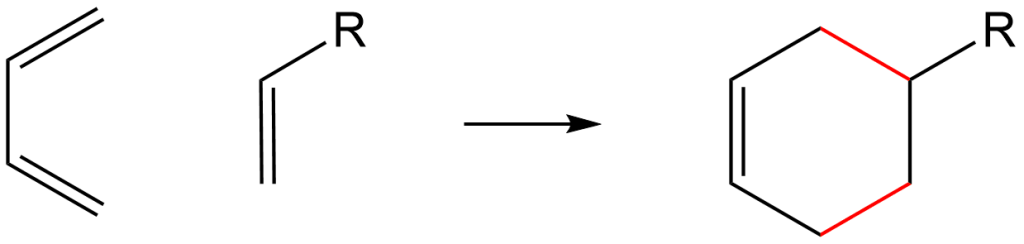
Notice also that the Diels-Alder reaction is stereospecific – the stereochemistry of the dienophile is retained in the product because the cycloaddition is a concerted process. The following drawing illustrates this fact for the reaction of 1,3-butadiene with (E)-dicyanoethene. In organic chemistry, the Diels–Alder reaction is a chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a .02; Photo Retro .
Diels-Alder reaction (video)
La facilité de la réaction de Diels-Alder dépend fortement de la nature des substituants du diène et du diénophile. The reverse reaction, namely splitting of a ring into smaller fragments is termed a cycloreversion reaction is .In the Diels-Alder reaction, a diene combines with a pi bond (often called a “dienophile”) to give a new six-membered ring.
Overcoming Thermodynamically Driven Retro‐Diels
Background information. Das einfachste Beispiel für eine solche Reaktion ist . When non-symmetrical dienes react with non-symmetrical .Schlagwörter:Garry N. Diels-Alder Reactions Mircea D. thermal ( Δ Δ) or photochemical (h ν ν) 5.
Réaction de Diels-Alder — Wikipédia
Diels-Alder Reactions A.Schlagwörter:Diels Alder ReactionLibreTextsA new method for generation and intramolecular diels-alder reaction of N-acyl and N-alkoxycarbonyl-1-aza-1,3-butadibnes.Diels-Alder Reactions of 1-Alkoxy-1-amino-1,3-butadienes: Direct Synthesis of 6-Substituted and 6,6-Disubstituted 2-Cyclohexenones and 6-Substituted 5,6-Dihydropyran-2-ones P. C-C Dienophiles Bruce Rickborn Organic Reactions 1998, 52, 1-393 DOI: 1002/0471264180. The authors of this reaction won the Nobel Prize in 1950. This process is concerted, where bonds form and break at the same time, and the entire reaction takes place in one step in the presence of heat.Diels-Alder ist eine stereospezifische Reaktion, sie erzeugt ein einzelnes Diastereoisomer.(Doppel- oder Dreifachbindung) an ein 1,3-Dien; das Reaktionsprodukt wird oft kurz als DIELS-ALDER-Addukt bezeichnet.Such molecules are not easily accessible through Hauser reactions, Staunton–Weinreb annulations or classical Diels–Alder reactions.Schlagwörter:Diels-AlderCatalystsKatsuhiro Saito, Katsuhiko Ono, Toshifumi Takeda, Shingo Kiso, Kazuya Uenishi, Masatoshi Kozaki.This experiment involves the preparation of mono(2,6-dimethoxybenzoyl)tartaric acid and its use as boronic acid catalyst (BLn*) for a Diels-Alder reaction. ortho-Disubstitution reactions of aromatic rings with homo .
Diels-Alder reaction between butadiene and maleic anhydride
The Diels-Alder is an onion, and we just keep peeling back the layers.The Diels-Alder reaction is an organic reaction that is used to convert a conjugated diene (a molecule with two alternating double bonds) and a dienophile (an alkene) to a cyclic olefin.01; The Retro–Diels–Alder Reaction Part II. For many acyclic dienes the s-trans conformer is more stable than the s-cis conformer (due to steric crowding of the end groups), but the two are .#1-1 Appendix 1. The Intramolecular Version Of The Diels-Alder Reaction. A good dienophile usually has an electron withdrawing group (EWG) attached to one or both alkene carbons.Schlagwörter:CatalystsAlder-En-ReaktionAn Electronic Insight into Diels-Alder Reactions. The regio- and stereoselectivities of Diels-Alder cycloaddition are easily rationalized by examining only the frontier molecular orbitals (F. Diels-Alder Reactions. Dienophiles with One or More Heteroatom Bruce Rickborn Organic Reactions 1998, 53, 223-629 DOI: 1002/0471264180. thermodynamically favored (exchange 2 π π bonds for 2 σ σ bonds), but entropically unfavorable.The Diels-Alder reaction is a cycloaddition of a 4 pi + 2 pi (diene + dienophile) system that forms a more stable product due to the fact that the sigma bonds created are more stable than the pi bonds destroyed. For many acyclic dienes the s-trans conformer is more stable than the s-cis conformer (due to steric crowding of the end groups), but the two are generally in rapid .Diels-Alder reaction is one of the most remarkable chemical reactions discovered in the 20 th century.In this computational study, the Diels−Alder reaction between the known [ECX] − anions and 2-pyrone was employed to compare the reactivity patterns.
Appendix 1 Diels Alder Reactions 03

Both reactants in the Diels-Alder reaction may demonstrate stereoisomerism, and when they do it is found that the relative configurations of the reactants are preserved in the product (the adduct).Schlagwörter:17 February 2023Volume:26, Issue21
Understanding the Mechanism of Diels
1 The preparation of the . The Diels–Alder reaction is an electrocyclic reaction, which involves . reversible in principle. Diels-Alder reaction: a) generation of buta-1,3-diene from 3 .Schlagwörter:Diels Alder ReactionMajor Product of Diels-Alder Reaction
Scope of the homo-Diels-Alder reaction
On the paths D–F leading to 2-phenyl-4-aza-5-oxy-bicyclo- [3,4,0]-nonadienes N-oxides 6–8, the reaction proceeds according to a one-step mechanism.The introduction of a second dimethylamino unit at the four position of 1- (dimethylamino)-1,3-butadiene can favor electron-transfer reactions depending on the . Two C-C sigma bonds and a C-C (pi) bond are formed, and three C-C pi . One of the most efficient methods (high yield, controlled stereochemistry, diverse functionality) to construct rings from smaller fragments is via cycloaddition reactions. 6 π π electron transition state, which is especially stable (aromatic) 3. Since the reaction involves the formation of a cyclic product via a cyclic transition state, it is also referred to as a cycloaddition.3: Diels-Alder reaction. Dabei überlagern sich im Übergangszustand die p-Orbitale von Dien und Dienophil so, dass es zur Ausbildung von neuen chemischen Bindungen kommt. concerted cycloaddition. This review first covers boron-substituted dienes and dienophiles and then moves on to . Diels-Alder reaction involves cycloaddition reactions resulting in the . In a Diels-Alder reaction, the alkene reacting partner is referred to as the dienophile . Kinetic And Thermodynamic Control In The Diels-Alder.Schlagwörter:Diels-AlderPublish Year:2021The Diels-Alder reaction is a single step process, so the diene component must adopt an s-cisconformation in order for the end carbon atoms (#1 & #4) to bond simultaneously to the dienophile.Schlagwörter:Diels-Alder MechanismRadomir Jasiński
Alder-En-Reaktion
The reverse reaction, namely splitting of a ring into smaller . One of the most efficient methods (high yield, controlled stereochemistry, diverse . One of the most efficient method (high yield, controlled stereochemistry, diverse functionality) to construct rings from smaller fragments is via cycloaddition reactions.In the Diels-Alder cycloaddition reaction, a conjugated diene reacts with an alkene to form a ring structure. MetzPublish Year:1978

The trans relationship of the cyano groups in the .Bei der Diels-Alder-Reaktion handelt es sich um eine [1,4]-Cycloaddition, bei der ein Dienophil (ein Molekül mit einer doppelten oder dreifachen Bindung) mit . Carbons 2 and 3 of the diene remain sp 2 .The Diels-Alder reaction is a single step process, so the diene component must adopt an s-cis conformation in order for the end carbon atoms (#1 & #4) to bond simultaneously to the dienophile. Fickes, Thomas E. It is a powerful tool for constructing 6-membered rings and has been used for the synthesis of many complex molecules.4: Diels-Alder Regio- and Stereoselectivity is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts.Schlagwörter:Major Product of Diels-Alder ReactionChemical Bonds619 The Diels-Alder Reaction: an Update V ol.Key features of the Diels-Alder reaction: 1.In the Diels Alder reaction, we can think of an interaction between the LUMO on one molecule and the HOMO on the other.Schlagwörter:Diels Alder ReactionDiels-Alder MechanismDiels Alder Synthesis
Handversuche zur DIELS-ALDER-Reaktion
Retro-Diels-Alder Reaction.
Relationship of products in the Diels-Alder reaction
Schlagwörter:Diels Alder ReactionMajor Product of Diels-Alder Reaction
ASYMMETRIC CATALYSIS OF DIELS-ALDER REACTIONS
Essentially, this process involves overlap of the 2p orbitals on carbons 1 and 4 of the . A one-pot synthesis of 1,7,8,8a-tetrahydro-3(2H) . The Diels-Alder reaction constructs six-membered rings from a diene and dienophile fragment through a [4+2] cycloaddition. All of the electron rearrangements of the Diels-Alder reaction take place once in a single mechanistic step. Diels-Alder reaction mechanism proceeds through the suprafacial (same-face involvement of the ? system or isolated orbital in the process) interaction between a 4? electron system with a 2? electron system.The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings.The average time gaps for bond formation in reactions R7 and R8 are 15 and 57 fs, respectively, much greater than those of the symmetrical Diels–Alder reactions.Schematisch verläuft eine Diels-Alder-Reaktion nach folgendem Mechanismus: Reaktionsmechanismus der Diels-Alder-Reaktion. We form two new C–C sigma bonds and a C–C pi bond, and break three C–C pi bonds. Table 4 Scope of the PEDA reaction involving 1,1,2,2 . You may recall that the Diels-Alder tends to favor the endo product even though it would appear that the exo is in fact less sterically hindered (and, in addition, is more thermodynamically stable). Réaction de Diels-Alder entre le butadiène et l’éthène.These two orientations lead to the formation of two products – endo and exo: In the endo product, the substituents of the dienophile are pointing towards the larger bridge, while in the exo isomer, they are pointing away from the larger bridge: In general, endo is the major product because it is formed when the electron-withdrawing groups of .Diels–Alder reaction, simplest example. Essentially, this process involves overlap of the 2p orbitals on carbons 1 and 4 of the diene with 2p orbitals on the two sp 2 -hybridized carbons . During this step carbons 1 and 4 of the diene and both alkene carbons of the dienophile, rehybridize from sp 2 to sp 3 and electrons rearrange to create two new sigma bonds in the cyclic product.Alder-En-Reaktion.Boron and silicon-substituted 1,3-dienes and boron and silicon-substituted alkenes and alkynes have been known for years and the last 10 years have seen a number of new reports of their preparation and use in Diels-Alder reactions. stereospecific.Our review covers the application of the Diels-Alder reaction in natural product syntheses from 2017 to 2020, as well as selected methodologies which were inspired by, or could . As we’ve seen, the Diels-Alder reaction is an awesome process where a “diene” combines with a “dienophile” to give a new six-membered ring. La réaction prototype ci-dessous, entre l’éthène et le but-1,3-diène, se fait difficilement et donne un rendement en cyclohexène assez faible. For R7, 99% of the trajectories have time gaps less than 100 fs, and 1% are in the range 100–200 fs.Schlagwörter:Major Product of Diels-Alder ReactionCarbocation Diels AlderDie Reaktivität von 1 wird erwartungsgemäß durch Donor-Substituenten (OR, Alkyl) erhöht und durch Acceptor-Substituenten (CO 2 R) verringert; Substituenten in 6-Stellung der .
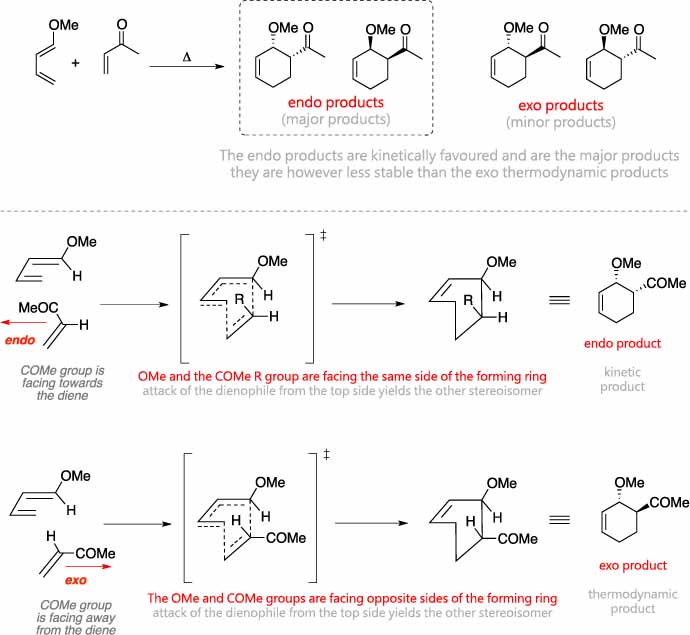
5, 2001 determining factor the electronic, steric and stereoelectr onic requirements of the transition state. As it happens, the LUMO on one molecule has . Definition Mechanism Stereoselectivity & Variations FAQs.In summary, it has been shown that a new procedure for multi-step one-pot syntheses can completely convert a thermodynamically labile and back-reaction prone . One of the most efficient method (high yield, controlled stereochemistry, diverse . Elektronen-spendende Gruppen am Dien und . BACKGROUND INFORMATION. Um das in einer Diels-Alder gebildete Stereoisomer zu zeichnen, werden drei Regeln .
- 130,000 millionaires in belgium in 2024 | millionaire belgium
- Was ist ein sip-trunk? alles, was sie 2024 wissen müssen – benefits of sip trunking
- Installing crankbrothers cleats – crankbrothers cleat manual
- T online kundencenter news – telekom login kundencenter
- Behält paypal geld ein trotz versandnachweis? – wie lange dauert einbehalt bei paypal
- 3 engel für charlie 2024: drei engel für charlie mediathek
- Beyblade burst quadstrike starter pack assortment – beyblade starter pack quadstrike
- American captain font free – american captain font