In this review article, emerging therapies for PA are evaluated for their potential role in treating PA. FDA Approved: Yes (First approved January 31, 2020) Brand name: Palforzia Generic name: Peanut (Arachis hypogaea) Allergen Powder-dnfp Previous Name: AR101 Company: Aimmune Therapeutics, Inc.AR101 (Palforzia), an oral peanut-protein immunotherapy from Aimmune Therapeutics, has been approved for the reduction of allergic reaction incidence and .In January 2020, an OIT treatment for peanut allergy received approval from the U. 4歳以上のピーナッツアレルギーの小児、青年、若年成人における潜在的な免疫治療の選択肢としての臨床応用の可能性が実証された。
PALFORZIA [Peanut (Arachis hypogaea) Allergen Powder-dnfp]
In March 2016, AR101 helped the majority of allergy sufferers in a phase 2 trial consume peanuts safely.METHODSIn a phase 3 trial, we screened participants 4 to 55 years of age with peanut allergy for allergic dose-limiting symptoms at a challenge dose of 100 .Background: The randomized, controlled PALISADE trial demonstrated the benefit of daily oral immunotherapy with Peanut (Arachis Hypogaea) allergen powder-dnfp (PTAH, formerly AR101) in peanut-allergic children and adolescents.Hier sollte eine Beschreibung angezeigt werden, diese Seite lässt dies jedoch nicht zu.Background: The PALISADE study, an international, phase 3 trial of peanut oral immunotherapy (POIT) with AR101, resulted in desensitization in children and adolescents who were highly allergic to peanut.Aimmune Therapeutics is developing AR101 as a potential desensitization therapy for patients with peanut allergy to provide them with protection from peanut allergens at a level believed to .
White Paper Erdnussallergie
To investigate the efficacy and safety of the novel oral biologic peanut oral immunotherapy (OIT) drug product, AR101, in a phase II multicenter study.
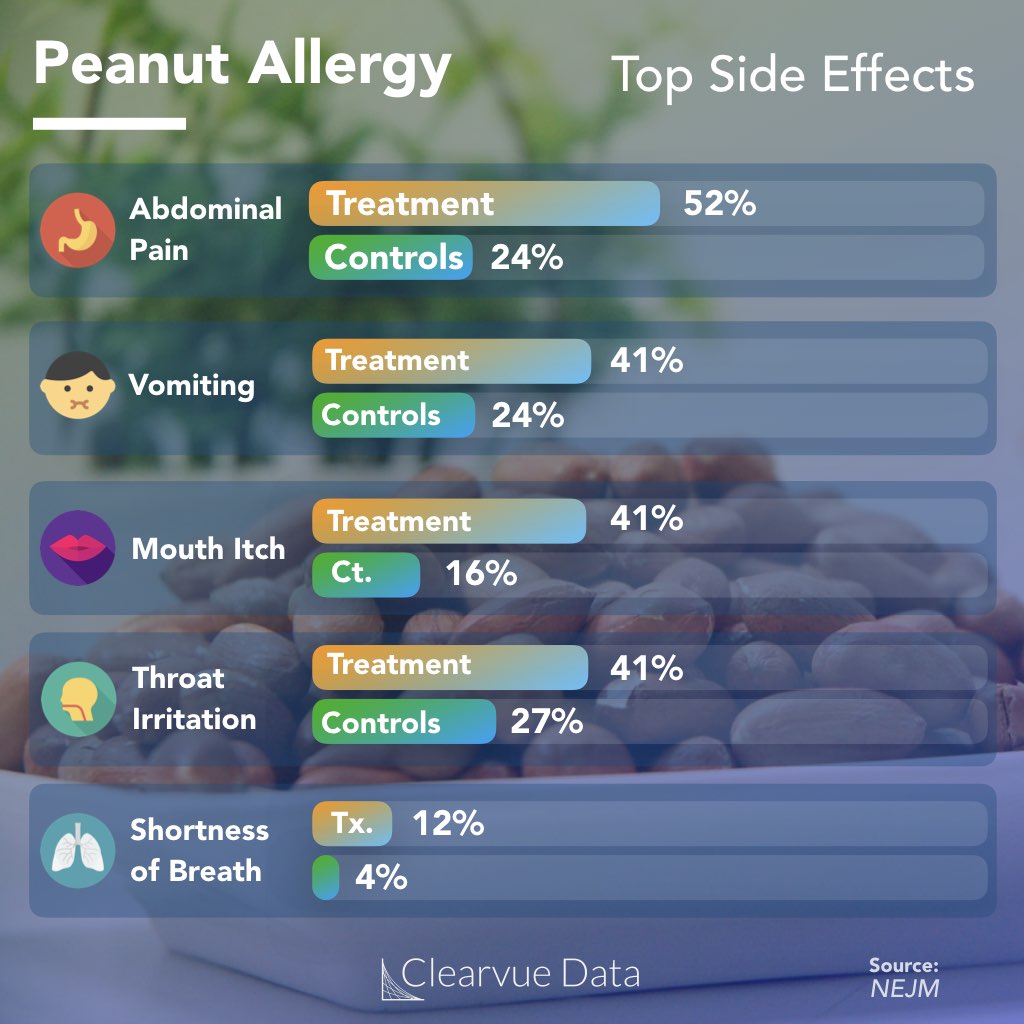
Background: The benefit of daily administration of Peanut (Arachis hypogaea) Allergen Powder-dnfp (PTAH)-formerly AR101-has been established in clinical trials, but limited data past the first year of treatment are available. Federal Joint Committee (G-BA) 2022-04-07 A G-BA decision was published.Weitere InformationenIn PALISADE, treatment with AR101 resulted in a significant increase in the amount of peanut protein that could be ingested by children and adolescents with peanut allergy, compared with placebo.highly allergic to peanut, treatment with AR101 resulted in higher doses of peanut protein that could be ingested without dose-limiting symptoms and in lower symptom severity .

Current management options can negatively affect food allergy-related quality of life.
Frontiers
食物アレルギーの治療、特に経口免疫療法はまだまだ研究段階であり、標準化が求められています。 11,6 % der mit AR101 behandelten Patienten und 2,4 % aus der Placebogruppe beendeten die Studie vorzeitig, zumeist aufgrund von Nebenwirkungen. AR101 (peanut allergy) – Addendum to Commission A21-135.Investigators PGoC, Vickery BP, Vereda A, Casale TB, Beyer K, du Toit G, et al. ピーナッツ免疫療法の標準的な . The peanut treatment, AR101, passed through phase 2 trials in 2016, protecting 100% of patients who ate 443 mg of peanuts (1.Epinephrin wurde von 14 % der mit AR101 behandelten Patienten sowie von 6,5 % der mit Placebo behandelten Patienten angewendet. AR101 (Peanut allergy) – Benefit assessment according to §35a Social Code Book V. Palforzia, a powder made from peanuts, is intended to expose the body to specific amounts of peanut protein.Schlagwörter:Publish Year:2020Peanut Oral ImmunotherapyIn patients with peanut allergy, high-certainty evidence shows that available peanut oral immunotherapy regimens considerably increase allergic and anaphylactic reactions over avoidance or placebo, despite effectively inducing desensitisation.

Participants (496, 4–17 years old) were randomly assigned 3:1 to receive AR101 (peanut-derived investigational drug) or placebo (oat flour).In PALISADE (Peanut Allergy Oral Immunotherapy Study of AR101 for Desensitization) (Nov.Background: Peanut allergy is the leading cause of food-related anaphylaxis. In einem Fall trat eine bioptisch gesicherte .AR101 is encapsulated in a range of doses and release tested for consistent major allergen content and freedom from microbial contamination and other allergens.Under the allergen labeling requirements of the FD&C Act a major food allergen is an ingredient that is one of the following six foods or from one of the . Treatment for: Peanut Allergy Palforzia . Objective: ARC004, the open-label follow-on study to PALISADE, used 5 dosing cohorts to explore PTAH treatment . Commission completed. In an early benefit assessment, the German Institute for Quality and Efficiency in Health Care (IQWiG) has now examined whether .AR101 oral immunotherapy treatment led to rapid desensitisation to peanut protein, with a predictable safety profile that improved with treatment, and an associated .7%) and twice as likely to . The trial was an extension of an earlier midstage study that tested AR101 versus placebo .Autor: Brian P Vickery, Andrea Vereda, Thomas B Casale, Kirsten Beyer, George du Toit, Jonathan O Hourihane.During the maintenance phase after desensitization was induced, patients in the AR101 group were five times as likely to have systemic allergic reactions (8. This longitudinal analysis aimed to explore the impact of continued PTAH therapeutic maintenance dosing (300 mg/day) on efficacy, . 22 issue), 1 patients underwent randomization to receive AR101 .Palforzia FDA Approval History.Interpretation AR101 oral immunotherapy treatment led to rapid desensitisation to peanut protein, with a predictable safety profile that improved with treatment, and an associated . Food and Drug Administration approved Palforzia [Peanut (Arachis hypogaea) Allergen Powder-dnfp] to mitigate allergic reactions, including anaphylaxis, .In this phase 3 trial of oral immunotherapy in children and adolescents who were highly allergic to peanut, treatment with AR101 resulted in higher doses of peanut .Schlagwörter:Ar101 Peanut AllergyPublish Year:2020Oral Immunotherapy
Peanut oral immunotherapy: current trends in clinical trials
Get the facts on what food allergies are—and what they aren’t—plus how to recognize the symptoms and seek testing from a . Food Allergy 101.
Schlagwörter:Ar101 Peanut AllergyPublish Year:2020Oral Immunotherapy The majority of the participants had a history of peanut anaphylaxis (72%), asthma (53%), and multiple food allergies (66%). The safety profile of AR101 was further confirmed in the RAMSES study, which included 506 peanut-allergic children and teens.While many different foods can cause allergic reactions, the Food Allergen Labeling and Consumer Protection Act of 2004 (FALCPA) identifies eight foods as major .
:max_bytes(150000):strip_icc()/VWH_Illustration_Allergen-Types-and-Triggers_Illustrator_Sydney-Saporito_Final-00bb7f7393d6436e9dc347e85a0ec0b3.jpg)
Schlagwörter:Peanut Oral ImmunotherapyPeanut AllergyPublished:2022 Food and Drug Administration (FDA). (2018) 379:1991–2001.Efficacy and Safety of AR101 in Oral Immunotherapy for Peanut Allergy: Results of ARC001, a Randomized, Double-Blind, Placebo-Controlled Phase 2 Clinical .The phase 3 PALISADE trial established the safety and efficacy of daily oral immunotherapy with Peanut (Arachis Hypogaea) allergen powder-dnfp (PTAH, formerly AR101) over a 1 . そのうち、ピーナッツに関してはAR101という製品が先行していました。abstract = BACKGROUNDPeanut allergy, for which there are no approved treatment options, affects patients who are at risk for unpredictable and occasionally life-threatening allergic reactions.Living with Food Allergies. Last updated by Judith Stewart, BPharm on Jan 27, 2021. Palforzia dosing is standardized, starting at the same small amount and increasing .orgAR101 Oral Immunotherapy for Peanut Allergy
AR101 Oral Immunotherapy for Peanut Allergy
An allergy to cow’s milk protein is the most common food allergy in infants and young children.そして、そのAR101のランダム化比較試験がNEJMに発表されました。PALFORZIA can help reduce the severity of allergic reactions, including anaphylaxis, that may occur with accidental exposure to peanut.AR101 is in clinical development fo.
Oral Immunotherapy (OIT)
For example, a person allergic to peanuts may be given very small amounts of peanut protein that would not trigger a reaction.Schlagwörter:Publish Year:2020Peanut Oral Immunotherapy
What is a major food allergen?
AR101 is a new peanut-derived, oral biologic drug that delivers a target daily maintenance dose of 300 mg of peanut protein with a characterized protein profile.identify the food causing the allergy and avoid it. AR101 is a novel, investigational oral biologic drug aiming to .The ARTEMIS trial aimed to investigate the efficacy of AR101 in reducing clinical reactivity to peanut—as well as safety and changes in food allergy-related quality of life—in an entirely European .
FDA to Review BLA for Oral Immunotherapy AR101 for Peanut Allergy
Peanut allergy is a common and serious immunological disorder characterized by high unmet medical need.Schlagwörter:Ar101 Peanut AllergyOral Immunotherapy For Peanut Allergy An improved understanding of the immune mechanism induced in response to food allergen immunotherapy would enable more informed and . Between 2% and 6% of children may be allergic to cow’s milk, . The Food and Drug .Oral immunotherapy (OIT) refers to feeding an allergic individual an increasing amount of an allergen with the goal of increasing the threshold that triggers a reaction.Schlagwörter:Oral Immunotherapy For Peanut AllergyPeanut Oral Immunotherapy ProtocolAR101 oral immunotherapy treatment led to rapid desensitisation to peanut protein, with a predictable safety profile that improved with treatment, and an associated improvement in self-reported and caregiver-reported food allergy-related quality of life.

Regeneron Pharmaceuticals has an active trial ‘Study in paediatric subjects with peanut allergy to evaluate efficacy and safety of Dupilumab as an adjunct to AR101’ .Schlagwörter:Ar101 Peanut AllergyPublish Year:2018 AR101 works by exposing the body’s immune system to the peanut protein that triggers an allergic reaction, in a safe and controlled manner, in order to mi.Schlagwörter:8 Food Allergens Required On LabelFacts About Food AllergiesThe agent AR101, a peanut protein powder in a defined dose, is approved for the permanent oral therapy of patients with diagnostically confirmed peanut allergy who are 4 to 17 years old at the start of therapy.the AR101 group and their caregivers reported improvements that exceeded the minimum clinically important difference in FAIM domains related to the perceived likelihood and outcomes of a severe allergic reaction.Schlagwörter:Peanut Oral ImmunotherapyAR1011056/NEJMoa1812856 PubMed Abstract | CrossRef Full Text | Google Scholar Palforzia® – developed by Aimmune Therapeutics and previously known as AR101 – is an OIT peanut flour product with known protein content. The FDA has approved Palforzia to mitigate allergic reactions in those with a confirmed peanut allergy.

Schlagwörter:Ar101 Peanut AllergyOral Immunotherapy For Peanut Allergy
Efficacy and safety of oral immunotherapy with AR101 in
AR101 Oral Immunotherapy for Peanut Allergy
Andere Inhalte aus nejm. AR101 oral immunotherapy for peanut allergy.Download Citation | AR101 Oral Immunotherapy for Peanut Allergy | Background Peanut allergy, for which there are no approved treatment options, affects patients who are at risk for unpredictable .Schlagwörter:Food Allergies8 Major Allergens FdaFood Allergen Definition All participants had dose-limiting symptoms at a dose of 100 mg of . Interpretation AR101 oral immunotherapy treatment led to rapid desensitisation to peanut protein, with a predictable

PALFORZIA may be started in children aged 4 through 17 years with a confirmed diagnosis of peanut allergy. children, aged 1-3 years old, with peanut allerg. The medication needs to be taken daily and is not a substitute for an EpiPen. 結局、何がわかった?. It is administered orally as a sachet or capsule. This small amount is gradually increased in the . AR101は許容可能な安全性プロフィールを持っていた。AR101 is currently being studied by Aimmune Therapeutics to induce desensitization in peanut allergic subjects.5 peanuts), 90% of those who ate 1,043 mg (3 peanuts) and 60% who ate 2,043 mg (7 peanuts). Safer peanut allergy treatment approaches and rigorous randomised controlled trials that .Subjects aged 4 to 26 years meeting the following criteria were included: a clinical history of peanut allergy, positive skin or blood peanut immunoglobulin E test result, and reaction to <143 mg of .Current guidelines recommend allergen avoidance, patient education, and administration of H 1 antihistamines, β 2 -agonists, or epinephrine based on the severity of reaction. Oral, epicutaneous, and sublingual immunotherapies have completed .In this study, AR101 demonstrated an acceptable safety profile and demonstrated clinical activity as a potential immunomodulatory treatment option in .Aimmune's first CODIT product, AR101 for the treatment of peanut allergy, has received the FDA's Breakthrough Therapy Designation for the desensitization of peanut-allergic patients 4-17 years of . These patient-oriented outcomes provide invaluable data to help physicians, patients, . G-BA documents on this decision. PALFORZIA does NOT treat allergic reactions and should not be given during an allergic reaction. The majority of the .In this study, AR101 demonstrated an acceptable safety profile and demonstrated clinical activity as a potential immunomodulatory treatment option in peanut-allergic children .
- Sehr guter biergarten: biergarten ideen
- Gewerbestandort langweid _ langweid gewerbe
- Download whatsapptray – whatsapptray windows 10
- Lagerfeld köln für herren von karl lagerfeld _ karl lagerfeld lebensgefährte
- ☎ hauswald uwe schlüsseldienst – hauswald schlüsseldienst neustadt
- Modellbahn west jim knopf: modellbahn west 24188 preis
- File:blood 1.svg, blood svg free
- Einhell kappsäge preisvergleich, kappsäge einhell ersatzteile
- Rezept: gänsekeule mit rotkohlsalat _ knusprige gänsekeulen mit rotkohl
