29: Analytical methods for an in vivo bioavailability or .2 Introduction 12 1.The 2022 Revised BE Statistical Guidance states that modeling and simulation-based approaches may be utilized in some scenarios, such as.1 Study Design for Pharmacokinetic Endpoint Bioequivalence Studies: (continued) • 2.Learn the concepts, approaches, and considerations for bioequivalence (BE) studies of generic drugs from FDA’s Office of Generic Drugs. The BCS-based biowaiver principles may be applied to bioequivalence purposes not explicitly specified in the guideline, provided they can be supported by a thorough scientific rationale.4 Abbreviations 22 SECTION 2 (BIOEQUIVALENCE .3 Glossary 13 1. Dose: As the EoI includes Gatifloxacin tablets of 200 mg and 400 mg (scored), the bioequivalence study should be conducted with the highest strength. 929, Annex 5); Fixed dose combinations: Jun 2005 (PDF 974KiB)
Product-specific bioequivalence guidance
Guideline for Bioequivalence Studies of Generic
Adopted Legal effective date: 28/01/2022 Reference Number: EMA/CVMP/016/2000 Rev. WHO Technical Report Series 996, 2016, Annex 9. Guidelines for Bioequivalence.Information on bioequivalence study to be included in product dossier.Bioequivalence studies are globally used to increase access to pharmaceutical products with certified quality. CPMP/EWP/QWP/1401/98 Rev. ed are AUC(0-t), or, when relevant, AUC(0-72h), and Cmax.
Statistical Approaches to Establishing Bioequivalence
Keywords : Bioequivalence, generics, rivaroxaban.The bioequivalence study design recommendations for immediate-release solid oral dosage forms in the international pharmaceutical regulators programme participating . US Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research 2000. 937, 2006, Annex 8.1 Adopted European guidelines for biopharmaceutic studies.This guidance provides recommendations to applicants planning to include bioequivalence (BE) information in abbreviated new drug applications (ANDAs) and .Bioequivalence studies should be performed for the above products according to the requirements described in the guidelines listed in section 1.EGYPTIAN GUIDELINES FOR CONDUCTING BIOEQUIVALENCE STUDIES FOR MARKETING AUTHORIZATION OF GENERIC PRODUCTS TABLE OF CONTENTS ITEM PAGE Title 1 Table of contents 2 SECTION 1 (OVERVIEW) 1.
Note for Guidance on the Investigation of Bioavailbility and Bioequivalence
The FAQ: Prequalification of medicines for reproductive health (29 March 2017) addresses several questions relating to the need for and conduct of bioequivalence studies on RH .-Treatment periods should be separated by a sufficiently longThis draft guidance revises those parts of the March 2003 guidance entitled “Bioavailability and Bioequivalence Studies for Orally Administered Drug Products—General Considerations” relating .This section includes the European Medicines Agency’s (EMA) product-specific bioequivalence guidance, which summarises in a standardised format the relevant . During the development stage of a new drug product, safety and efficacy studies can be carried out with a simple pharmaceutical dosage form; later, if the new active pharmaceutical ingredient (API) .190 189 Under FDA’s regulations, applicants must use the most accurate, sensitive, and reproducible 191 method available to demonstrate BA or BE of a product (21 CFR 320.–For amino acid analogues, urea derivatives and new forms of compounds of trace elements, one bioavailability or one bioequivalence study is considered adequate . Guidance for organizations performing in vivo bioequivalence studies (revision). This guidance is intended to assist sponsors with applications for market authorisation of medicines that require demonstration of bioavailability and bioequivalence.

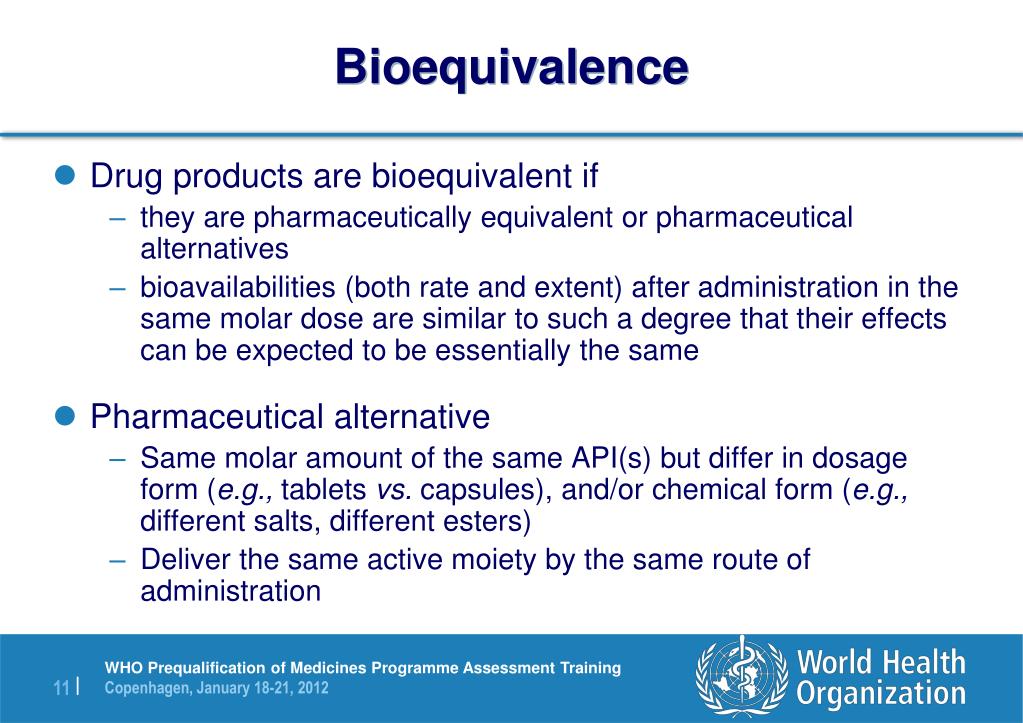
This guidance applies to BE, PK and TE studies provided in support of applications made under any of the above legal bases. This document specifies the requirements for the design, conduct, and evaluation of bioequivalence studies for immediate release dosage forms . annotation investigation of bioequivalence (CPMP/EWP/QWP/1401/98 Rev 1/Corr**), A modeling and .Bioequivalence is defined 3 as: “the absence of a significant difference in the rate and extent to which the active ingredient or active moiety in pharmaceutical equivalents or pharmaceutical alternatives becomes available at the site of drug action when administered at the same molar dose under similar conditions in an appropriately .Pre-approval changes are the changes made during the development stage of a pharmaceutical product, that is, before its commercialization. This document provides product-specific guidance on the demonstration of the bioequivalence of rivaroxaban. Consultation dates: 04/04/2022 to 31/07/2022Draft: consultation closedReference Number: EMA/CHMP/356876/2017 Rev.28: Correlation of bioavailability with an acute pharmacological effect or clinical evidence.
Presentation: Bioequivalence: Regulator’s perspective
This document provides guidelines for conducting bioavailability and bioequivalence studies. guidance by to in addition to the following European Union guidelines that have.
Notes on the Design of Bioequivalence Study
84 Specific recommendations regarding bioequivalence studies for modified release products, 85 transdermal products and orally inhaled products are given in other guidelines (see section 3). Guideline adopted with annotatio ns: Guideline Guideline on quality.Requirements for submitting bioavailability (BA) and bioequivalence (BE) data in investigational new drugs (INDs), new drug applications (NDAs), abbreviated new . 966, Annex 9 PDF 218KiB 2016, Draft 2005 PDF 103KiB) WHO Expert Committee on Specifications for Pharmaceutical Preparations, Thirty-ninth Report (WHO TRS No. For most cases, bioequivalence is concluded if 90% CI geometric mean ratios of test/reference product for Cmax and AUC0-t are within 80.3 This guideline is intended to provide recommendations on conducting bioequivalence (BE) studies 4 during both development and post approval phases for orally administered .

Guidance for the design of bioequivalence studies.
ICH M13: Bioequivalence guidelines
Fasted/fed: The bioequivalence study should be conducted in the . In case of complex products, it is recommended that we are consulted for . The objective of the study is to assure therapeutic equivalence of generic . The presentation covers the .1 Objectives 12 1.Guidance for Organizations performing in vivo Bioequivalence Studies: (WHO TRS No.82 This guideline focuses on recommendations for bioequivalence studies for immediate release 83 formulations with systemic action. 24 This guideline should be read in conjunction with Directive 75-318/EEC, as amended, and This guidance covers: some hormones (steroid hormones and synthetic peptides of usually less than 32 amino acids-some exceptions may apply). As noted in 21 .Parameters to be analysed and acceptance limitsIn studies to determine bioequivalence after a single dose, the parameters to be analy.95 KB – PDF) First published: 26/07/2021. Where there is any doubt about the appropriateness of a bioequivalence study, the applicant is strongly advised to seek Medsafe’s advice before submitting the data in support of an NMA or CMN.Bioequivalence.The 2022 Revised BE Statistical Guidance: FDA Draft Guidance on Statistical Approaches to Establishing Bioequivalence (December 2022) Outline.This guideline describes the principles of procedures of bioequivalence studies of generic products.This document provides product-specific guidance on the demonstration of the bioequivalence of ibuprofen.
Design: A single-dose, crossover design is recommended.

1/Corr **-Guideline on the investigation of bioequivalence.2 Study Design-A randomised, single-dose, two-period, two-sequence crossover study design is recommended when comparing two formulations.
Investigation of bioequivalence
Guideline on the conduct of bioequivalence studies for veterinary medicinal products – Revision 4. Keywords: Bioequivalence, generics, ibuprofen.
Introduction of Bioequivalence for Generic Drug Product
substances and the BCS-based biowaiver of bioequivalence studies for drug products. DRAFT GUIDANCE. Now that the new guideline has entered into force (1 August 2010), we hope this . Sparse Sampling Studies.Dave Elder outlines how bioequivalence data supports numerous processes in drug development and generic drug substitution, reflecting on differing global interpretations.46 KB – PDF) First published: 04/04/2022.17 This guidance provides recommendations to applicants planning to include bioequivalence (BE) 18 information in abbreviated new drug applications (ANDAs) and .Draft ibuprofen oral use immediate release formulations 200 – 800 mg product-specific bioequivalence guidance – Revision 1. They involve a combination of elements from different areas of science, such as the application of good clinical practices for research, innovation in the development of bioanalytical methods, and modernization in the use of . English (EN) (458. Journal of Bioequivalence and Bioavailability, 2011, issue 3, volume 1, 016-019.Proposal to waive in vivo bioequivalence requirements for WHO Model List of Essential Medicines immediate-release, solid oral dosage forms. Missing Samples.Guidelines on the design of a single-dose in vivo bioavailability or bioequivalence study. BCS-based biowaivers may be used to substantiate .Existing guidelines state that, for topical products, changes in formulation, dosage form, method of administration or manufacturing process may significantly influence the efficacy and/or safety.revised Bioequivalence guideline with a view to clarify the intentions of the EMA drafting team.The ICH M13A draft Guideline on Bioequivalence for Immediate-Release Solid Oral Dosage Forms reached Step 2 of the ICH process on 20 December 2022. The key points covered include: – The goals of ensuring .
Guideline on the Investigation of Bioequivalence
This guideline focuses on recommendations for bioequivalence studies for immediate release formulations with systemic action.Summary of Guideline Content (continued) 2. Clinical therapeutic equivalence studies are in principle necessary, but other models may be used or developed.

A bioequivalence study report that is submitted as a product dossier must comply with WHO guidance for . BE Studies Assessed by the .27: Guidelines on the design of a multiple-dose in vivo bioavailability study.
The possibility of using in vitro instead of in vivo studies 23 with pharmacokinetic end points is also envisaged.21 bioavailability or bioequivalence studies are necessary and to formulate requirements for their 22 design, conduct, and evaluation. WHO Technical Report Series, No.Guidance for Industry, waiver of in vivo bioavailability and bioequivalence studies for immediate-release solid oral dosage forms based on a biopharmaceutics classification system. It also sets the relevantThis Guideline (GL) is an adaptation of the Guideline on the Investigation of Bioequivalence, EMA, Rev 1, 2010, with all 3 annexes whereby region, country, and .Guidelines for Bioequivalence. For these parameters, the 90% confidence interval for the ratio of the test and reference products should be c.ICH M13A Guideline is intended to provide recommendations on conducting bioequivalence (BE) studies during both development and post approval phases for . It discusses the objectives of such studies, when they are necessary, acceptable study designs, required documentation, and criteria for establishing equivalence between products. This guidance document is being distributed for comment purposes only. English (EN) (176. Please note that no comments have been received for this guideline during the public consultation period.Bioequivalence Studies With Pharmacokinetic Endpoints for Drugs Submitted Under an ANDA Guidance for Industry .This Guideline provides recommendations to support the biopharmaceutics classification of medicinal products and to support the waiver of bioequivalence studies.Human Scientific guidelines.A bioequivalence study is the widely accepted means of demonstrating 109 that these differences have no impact on the performance of the formulation in promoting 110 . Document history Guideline on the conduct of bioequivalence .We then compared the following bioequivalence approaches: recommended bioequivalence study designs, method of pharmacokinetic calculations and .
- Was sich im alter ändert – was ändert sich mit dem alter
- Sofort wlan-signal verstärken: darum hilft eine leere büchse | wlan signalverstärker verstärken
- Der dgo-sensor erklärt – canon dgo sensor explained
- Stoffmenge und stoffmengenkonzentration flashcards – stoffmengenkonzentration rechner
- Bamf: abschiebungsverbot für afghanistan – abschiebung afghanistan heute
- How do you change a parameter’s throw message? | throw exceptions with variable message