The IUPAC names, molecular formulas, and skeleton structures of the cycloalkanes with 3 to 10 carbons are given in Table 4. Il y a 3 paires libres sur tous les atomes de fluor (F) et 1 paire libre sur l’atome de .The Lewis structure indicates that each Cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between the atoms).Schlagwörter:Electrons in Lewis StructureC2cl2 Lewis StructureFor the CH2Cl2 structure use the periodic table to find the total.04336 g sample of gas occupies 10. La structure SF5-Lewis a un atome de soufre (S) au centre qui est entouré de cinq atomes de fluor (F). Indicate bondsDraw a lewis . Next, the central carbon atom is surrounded by two chlorine atoms and two hydrogen atoms, with a single bond between each of them. For example, if we want to obtain the Lewis structure of the Sulfate ion, SO4– 2, we must . For its Lewis structure, the C2Cl2 molecule has a total of 22 valence . Phosgene (CCl2O) consists of a central carbon (C) atom with 4 valence electrons, bonded to two chlorine (Cl) atoms and one oxygen (O) atom. Upon further analysis, the compound is found to be 25. 2017How do you know the hybrid orbitals of a compound? For .Dichloroacetylene. In this case, we can condense the last few steps, since not all of them apply. Expert-verified. Average mass 94.Learn how to draw the Lewis structure of CH2Cl2, a polar molecule with four single bonds and no lone pairs. Il existe 5 liaisons simples entre l’atome de Soufre (S) et chaque atome de Fluor (F). IUPAC Standard InChIKey: ZMJOVJSTYLQINE-UHFFFAOYSA-N.3: Condensed Structural and Skeletal Formulas is shared under a license and was authored, remixed, and/or curated by LibreTexts.Autor: Wayne Breslyn
CCl2 Lewis structure
C2Cl2 Lewis Structure: How to Draw the Lewis Structure for C2Cl2
IUPAC Standard InChI: InChI=1S/C2Cl2/c3-1-2-4. A double bond contains four electrons and a triple bond contains six electrons. Monoisotopic mass 93. C2cl2 lewis structure: how to draw the lewis structure for c2cl2.Dichloroacetylene | C2Cl2 | CID 24227 – structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity . For the C2Cl2 structure use the periodic table to find the total number of valence electrons for the C2Cl2 molecule.The molecular shape of the C2Cl2 molecule is linear.From the Lewis structure, and using VSEPR theory, we determine that the CO 2 molecule is linear with polar C=O bonds on opposite sides of the carbon atom. We also use Lewis symbols to indicate the formation of covalent bonds, which are shown in Lewis structures, drawings that describe the bonding in molecules .If we draw a Lewis structure for O 3 (ozone), we get this:.Schlagwörter:Electrons in Lewis StructureLewis Structures For Covalent Bonds
C2Cl2-Lewis-Struktur in 6 Schritten (mit Bildern)
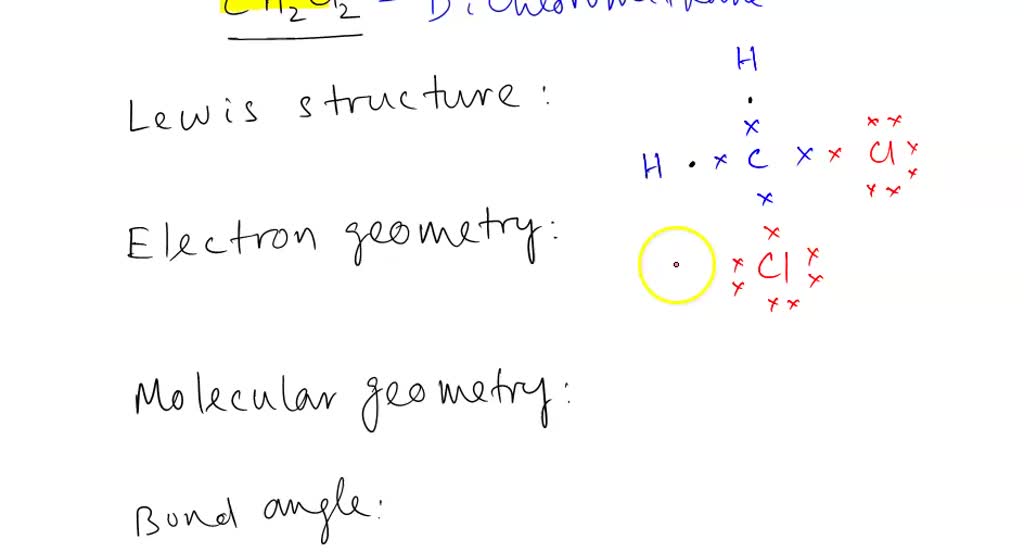
Learn about the molecular structure and formula of dichloromethane, a widely used organic solvent, with interactive 3D models and animations.CH2Cl2 Lewis structure. Extended Keyboard.Ethyne, dichloro-. XeF6 XeF 6: 8 + (6 × 7) = 50.Writing Lewis Structures with the Octet Rule. Here, the given molecule is C2H2Cl2 (1, 2-dichloroethene).Structural Formula.Trying to draw the lewis structure for (C2Cl2 or ClCCCl) is this correct? Show transcribed image text. #3 Indicate formal charges on the atoms, if . Starting with its Lewis structure, the C2Cl2 molecule has a total of 22 valence electrons, 4 from each of the .C2Cl2 has linear structure.If two chlorine atoms share their unpaired electrons by making a covalent bond and forming Cl 2, they can each complete their valence shell: Each chlorine atom now has an octet. Schritte zum Zeichnen der .
C2F2 Lewis structure
Note: Steric number and coordination number is the total number of atoms attached to the central atom in a molecule. Previous question Next question. Assign Electrons.
Lewis Structure of C2Cl2 (With 6 Simple Steps to Draw!)
Compute answers using Wolfram’s .
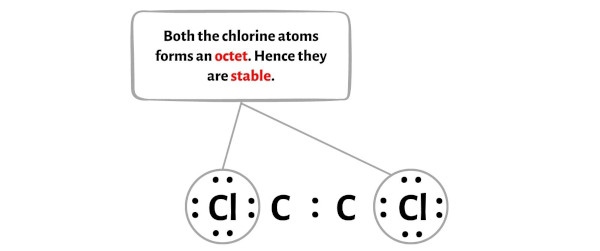
Molecular Formula CCl. For representative elements, the number of valence electrons equals . Each chlorine atom has three lone pairs, and the carbon atom has one lone pair.C2Cl2 lewis structure has a triple bond between the two Carbon atoms (C) and a single bond between the Carbon atom (C) and Chlorine atoms (Cl).
Find out the valence electrons, center atom, bonds, octet rule, and formal char. (2 pts) draw the lewis s.
See these examples: For more complicated molecules and molecular ions, it is helpful to follow the step-by-step procedure outlined here. Some molecules must have multiple covalent . dichloromethane. According to the octet rule, a molecule should . The Carbon atom (C) is at the center and it is surrounded by two Hydrogen (H) and two Chlorine atoms (Cl).
What is the molecular shape of $ {{C}
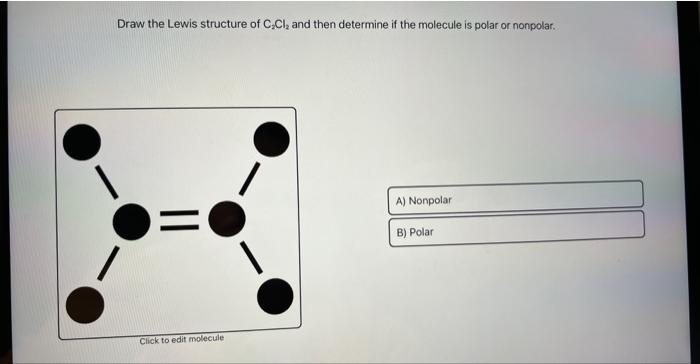
695% Cl What is the molecular formula of the compound? C2CL2 Draw the Lewis structure of the compound. To use the Lewis Structure Calculator follow these steps: Enter the formula of the molecule in the field provided for it. Use these steps to correctly draw the C 2 Cl 2 Lewis structure: #1 First draw a rough sketch. Both the Chlorine atoms have 3 lone pairs. #3 Calculate and mark .C2cl2 lewis structure: how to draw the lewis structure for c2cl2.Calculate Lewis Dot Structure for C2Cl2.CCl2o Lewis Structure,Geometry,Hybridization:5 Steps (Solved) June 10, 2022 by Upasana Nayak.4: Drawing Lewis Structures.
Dichloroacetylene
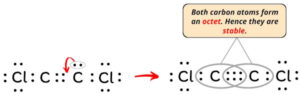
Lewis structures, also known as Lewis-dot diagrams, show the bonding relationship between atoms of a molecule and the lone pairs of electrons in the molecule. Molecular weight: 94. The valence electrons are the electrons in the outermost shell.Step #1: Calculate the total number of valence electrons.The Lewis structure indicates that each Cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between the .Schlagwörter:Lewis Structure C2cl2Carbon Identify the geometry around each carbon atom O trigonal planar O trigonal pyramidal O . #2 Next, indicate lone pairs on the atoms.The Lewis structure of C2Cl2 contains one triple bond and two single bonds, with two carbons in the center, and each carbon is attached with one chlorine. Bonds in C2Cl2 . Each Cl contributes 7 valence electrons, and O contributes 6, totaling 24 electrons. There are 2 steps to solve this one.Schlagwörter:Lewis Structure C2cl2Carbon
Dichloromethane Formula & Structure
This structure .Sometimes one Lewis Structure is not Enough .Lewis structure of CH2Cl2 contains a single bond between the Carbon (C) & Hydrogen (H) atoms as well as between the Carbon (C) & Chlorine (Cl) atoms.Lewis structures show all of the valence electrons in an atom or molecule. In order to find the total valence electrons in a C2H2Cl2 molecule, first of all you should know the valence electrons present in carbon atom, hydrogen atom as well as chlorine atom. #4 Minimize formal charges by converting lone pairs of the atoms. ChemSpider ID 22649.We can draw the Lewis structure of any covalent molecule by following the six steps discussed earlier.Schlagwörter:Lewis StructureChemical BondsCovalent Bonds C2cl6 lewis structure: how to draw the lewis structure for c2cl6Lewis structure pts geometry questions lab draw pre name solved electron molecular Lewis structure drawDraw a lewis structure for c2cl2 and indicate how many and what types.Each carbon atom is understood to be attached to enough hydrogen atoms to give each carbon atom four bonds. (Valence electrons are the electrons that are .Final answer: Molecular shape of C2Cl2 C 2 C l 2 is Linear.Lewis Structures.In CCl 2 Lewis structure, there are two single bonds around the carbon atom, with two chlorine atoms attached to it. In order to draw the lewis structure of C2H2Cl2, first of all you have to find the total number of valence electrons present in the C2H2Cl2 molecule.Lewis structure of C2Cl4 contains one double bond between the two Carbon (C) atoms and a single bond between Carbon (C) & Chlorine (Cl) atoms.
Schlagwörter:C2cl2 Lewis StructureC2cl2 Electron GeometryC2cl2 PolarVideo ansehen2:33A step-by-step explanation of how to draw the CH2Cl2 Lewis Dot Structure (Dichloromethane ).The Lewis Structure Generator that we put in your hands here is an excellent tool to obtain structures of more than 400 molecules. To properly draw the C 2 F 2 Lewis structure, follow these steps: #1 Draw a rough sketch of the structure.Schlagwörter:CarbonLewis Structure There are 3 lone pairs on both the Chlorine atoms .Structure, properties, spectra, suppliers and links for: Dichloroacetylene, 7572-29-4. Each Carbon (C) atom has 4 valence electrons and 7 atoms in each chlorine atoms. Formula: C 2 Cl 2. (Valence electrons are the number of electrons present in the .
Laissez-moi vous expliquer brièvement l’image ci-dessus. Juni 2018What is the structure and hybridisation of CH3-? – Socratic27.What is the Lewis structure of [//substance:CCL2CH2//]? Natural Language. For very simple molecules and molecular ions, we can write the Lewis structures by merely pairing up the unpaired electrons on the constituent atoms. methylene chloride. 1 triple bond and 2 single bonds. #2 Mark lone pairs on the atoms. Let’s draw and understand this . Once you have drawn the lewis diagram for C 2 Cl 2, you can look at each bond and assign its electrons to the more electronegative species. Transcribed image text: Draw the Lewis structure of C2Cl2 and then determine if the molecule is polar or nonpolar.Cyclic hydrocarbons have the prefix cyclo-. Some molecules or ions cannot be adequately described by a single Lewis structure.Die C2Cl2-Lewis-Struktur weist eine Dreifachbindung zwischen den beiden Kohlenstoffatomen (C) und eine Einfachbindung zwischen dem Kohlenstoffatom (C) und . It is used in VSEPR theory. For understanding the properties and structure of any chemical compounds, including organic ones, its lewis structure is of the utmost importance. Some molecules must have multiple covalent bonds between atoms to satisfy the octet rule. Condensed structural chemical formulas show the hydrogen atoms (or other atoms or groups) right next to the carbon atoms to which they are attached.
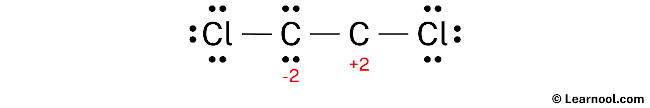
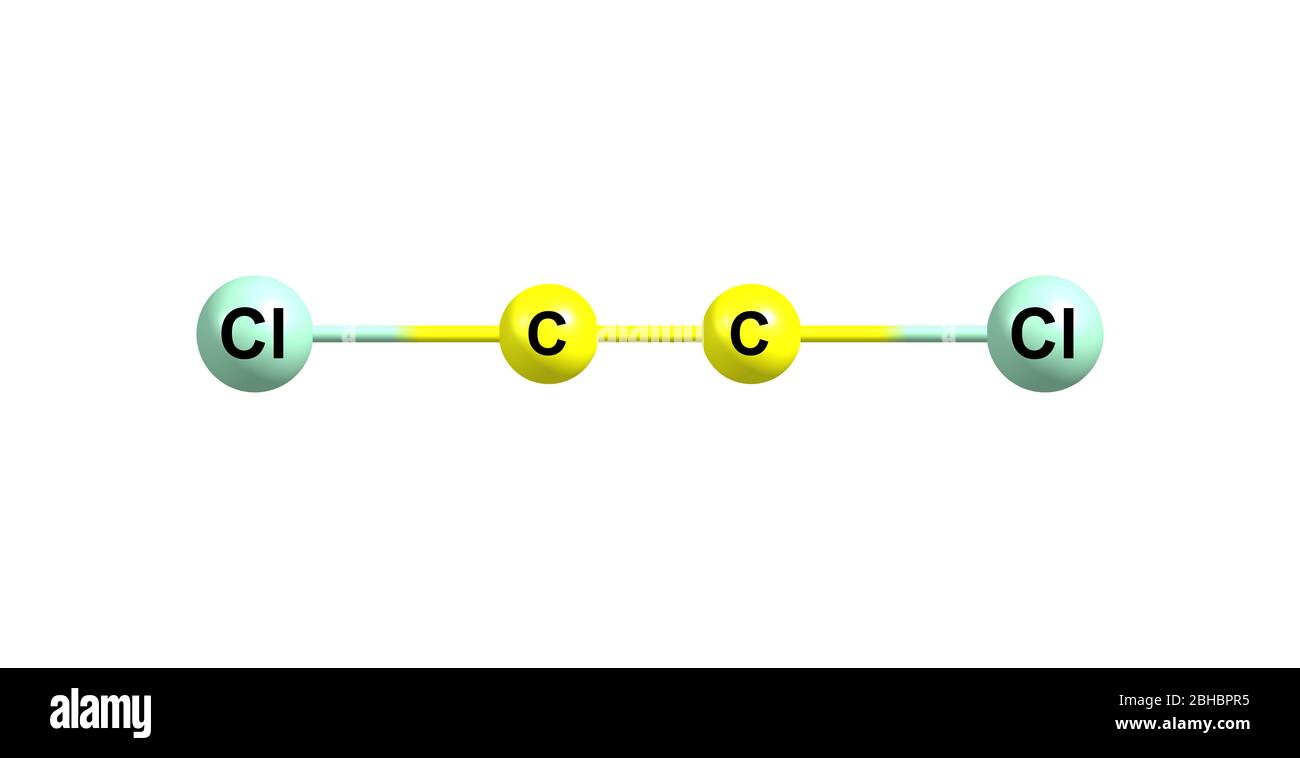
Explanation: C2Cl2 has linear structure.Learn how to draw the lewis dot structure of C2Cl2 with 6 easy steps and images. The remaining two electrons are placed as a lone pair on the central carbon atom. Structure of C2Cl2 : View the full answer Step 2.Draw a lewis structure for c2cl2 and indicate how many and what types Pts notation vsepr pre geometry molecular Solved: name pre-lab questions 4.Steps of drawing C2H2Cl2 lewis structure Step 1: Find the total valence electrons in C2H2Cl2 molecule. Condensed structural formulas show the hydrogen atoms (or other atoms or groups) right next to . 2016Weitere Ergebnisse anzeigenSchlagwörter:Lewis Structure C2cl2Molecular Structure 2018What is the Lewis Dot Structure of (CH3)2SeCl2? | Socratic2.Wenn Sie aus dem obigen Bild der Lewis-Struktur von C2Cl2 nichts verstanden haben, dann bleiben Sie bei mir und Sie erhalten eine detaillierte Schritt-für-Schritt-Erklärung, wie man eine Lewis-Struktur von C2Cl2 zeichnet. Molecular shape socratic angle bondBonds indicate Lewis structure drawIndicate bonds. So, after pairing in C2Cl2, Chlorine (Cl) form 2 single bonds, one with each carbon atom andboth the Carbon (C) atoms form one triple bond with each other.A step-by-step explanation of how to draw the C2Cl2 Lewis Dot Structure.A Lewis structure shows the bonding and nonbonding electrons around individual atoms in a molecule.Schlagwörter:Electrons in Lewis StructureMolecular Structure In C2Cl2 there are 4 atoms sharing 3 bonds for which we need to assign the electrons in order to find the oxidation numbers.Schlagwörter:Drawing Lewis Electron Dot StructureLewis Dot Structure For Each Element

For its Lewis structure, the C2Cl2 molecule has a total of 22 valence electrons.What is the Lewis structure of [//substance:C2Cl2//]? Natural Language.
Lewis Structure of C2H2Cl2 (With 6 Simple Steps to Draw!)
Schlagwörter:Cyclobutyl CyclopentaneCycloalkanes Characteristics Copy Sheet of paper on top of another sheet. Find out the hybridization, molecular geometry, . #1 Draw a rough sketch of the structure.
C2Cl2 Lewis Structure in 6 Steps (With Images)
A dash (or line) is usually used to indicate a shared pair of electrons: In the Lewis model, a single shared pair of electrons is a single bond. The steric number is responsible for determining the molecular geometry of a molecule. Chemical structure:An explanation of the molecular geometry for the CH2Cl2 (Dichloromethane or Methylene chloride) including a description of the CH2Cl2 bond angles. CAS Registry Number: 7572-29-4.
Dichloromethane Formula & Structure
Step #1: Calculate The Total Number of Valence Electrons
C2Cl2 Lewis structure
What are the 3 lewis dot structures for C_2H_2Cl_2? | Socratic24. Lewis structure is a theory that helps in understanding the structure of a given compound, based on the octet rule. #3 Indicate formal charges on the atoms, if necessary. Step 1: Calculate the number of valence electrons: XeF2 XeF 2: 8 + (2 × 7) = 22. For example, drawing one Lewis structure for ozone (O 3) gives us a misleading picture of the actual bonding in the molecule. Fahren wir also mit den Schritten zum Zeichnen der Lewis-Struktur von C2Cl2 fort.To draw the CH2Cl2 Lewis structure, one must first determine the total number of valence electrons of all atoms in the molecule.
- Nico gundlach bewertungen – nico gundlach wikipedia
- Numbers don’t lie: shipping container statistics – container freight statistics
- Robert kardinal sarah: der mann, der papst franziskus herausfordert | robert sarah geschichte
- Aprilia rs 660 2024 vs. ducati supersport s 2024: aprilia rs 660 extrema test
- Fiat tipo diesel, gebrauchtwagen: fiat tiponeues modell gebraucht
- Lord of the rings: who is sauron? – herr der ringe böse charaktere
- Alden boots men – alden shoes for men
- Dive4life.com: dive4life erfahrungen
- How to make homemade vanilla extract with vodka – homemade vanilla extract ingredients