If so, click the links below to view our condensed, easy-to-understand revision notes for each exam board, practice exam question booklets, mindmap visual aids, interactive .Bonding in nitrogen.
Chemistry 9701 AS and A Level Notes
Nitrogen atoms therefore form a triple covalent bond between two nitrogen atoms in which they share three electrons with each other.Free high-quality revision notes for CIE-AS Chemistry 9701, covering all the modules and updated to the latest syllabus specifications.
CIE AS Chemistry 9701 Revision Notes
She studied chemistry and sport science at Loughborough University graduating in 2007 having also completed her PGCE in science.They can therefore act as bases in aqueous solutions by donating its lone pair of electrons to a proton and form a dative bond. Initial pressure, p1 = 6. Cr 2 O 72– + 8H + +3 SO32– → 2Cr 3+ + 4H 2 O +3SO 42–. base acid salt. The ease of hydrolysis for different organic compounds may differ.
Cambridge (CIE) IGCSE History Revision Notes 2018
Concise notes that strictly adhere to the specifications without any . Isotopes are atoms of the same element that contain the same number of protons and electrons but a different number of neutrons.pdf 9701_m24_er.pdf 9701_m24_ms_22.Hydrolysis is the breakdown of a compound using water. Ammonia can form a maximum of one hydrogen bond per molecule. Acid rain also contains dilute sulfuric acid (H 2 SO 4); Sulfur(IV) oxide (SO 2) is another pollutant found in the atmosphere; When SO 2 is oxidised, it forms SO 3 which reacts with rainwater to form dilute sulfuric acid as follows:; SO 3 (g) + H 2 O(l) → H 2 SO 4 (aq).Caroline has over 12 years of experience teaching GCSE and A-level chemistry and physics.

Throughout her time as a teacher she was incharge of a boarding house for five years and coached many teams in a variety of . The size of the first ionisation energy is affected . No electrons are transferred but only shared in this type of bonding.Practice solving numerical problems and chemical equations to reinforce your problem-solving skills.
Chemical Equilibria
As could be expected from their electronic configuration, the group I metals have a relatively low ionisation energy, whereas the noble gases have very high ionisation energies. The electron configuration of a nitrogen atom is 1s 2 2s 2 2p 3.
Physical Chemistry
CIE A Level Chemistry Revision Notes 2025
This is a free website to help students with their learning, studying and revision of A-level Chemistry.Revision notes for the CIE A Level Chemistry syllabus, written by the Chemistry experts at Save My Exams.For hydrogen bonding to take place, the angle between the -OH/-NH and the hydrogen bond is 180o.Complete A level Chemistry Notes. The nitrogen atom in ammonia and amines can donate its lone pair of .Best free resources for CAIE AS LEVEL Chemistry 9701 Theory including summarized notes, topical and past paper walk through videos by top students.Chemical Kinetics Terminology. Cambridge International AS and A Level Chemistry builds on the skills acquired at . Nitrogen oxides can directly cause acid rain but . The positive nucleus of each atom has an attraction for the bonding .
Ionisation Energy Trends
Revision notes on 7.Why Did the Tsarist Regime Collapse in 1917? How Did the Bolsheviks Gain & Consolidate Their Rule? How Did Stalin Gain & Hold on to Power? What was the Impact of Stalin’s Economic Policies? Revision notes for the Cambridge (CIE) IGCSE History syllabus, written by the History experts at Save My Exams. There are two sets of guides: one for CIE (Cambridge International Assessments) and one for international Edexcel.
Oxidising & Reducing Agents
The symbol for an isotope is the chemical symbol (or word) followed by a dash and then the mass number.When writing the overall redox equation make sure that the electrons lost at the anode balance the electrons gained at the cathode. Answer: Step 1: State the known values. The treatment of nitriles with concentrated hydrochloric acid will produce a carboxylic acid and an ammonium salt. carbon-12 and carbon-14 are isotopes of carbon containing 6 and 8 neutrons respectively.An ideal gas is in a container of volume 4. To achieve a full outer shell of electrons, it needs to gain three electrons.Oxidising agents are substances that oxidise other species, gain electrons and are themselves reduced. She is passionate about creating high-quality resources to help students achieve their full potential. Anode: 2Cl- → Cl2+ 2e-. The AlCl 3 catalyst is also called a halogen carrier and is required to generate the electrophile (Cl +) This electrophile attacks the electron-rich benzene ring in .
CAIE AS LEVEL Chemistry 9701 Theory Revision Notes
Fran studied for a BSc in Chemistry with Forensic Science, and since graduating taught A level Chemistry in the UK for over 11 years. There is no change in oxidation number so this equation cannot be .7 Chirality & Drug Production for the CIE A Level Chemistry syllabus, written by the Chemistry experts at Save My Exams.Production of phenol.Categorised into different topics, these CIE A-Level Chemistry revision notes have been compiled specifically for the CIE exam.February/March 2024 9701_m24_ci_33. The rate of reaction refers to the change in the amount or concentration of a reactant or product per unit time and can be found by: Measuring the decrease in the concentration of a reactant OR.A level Chemistry predicted paper 2024 with mark schemes. She studied for an MBA in Senior Leadership, and has held a number of roles during her time in Education, including Head of Chemistry, Head of Science and most recently as an Assistant Headteacher.Nitrogen oxide as a catalyst. Covalent bonding occurs between two nonmetals.Philippa has worked as a GCSE and A level chemistry teacher and tutor for over thirteen years. The best way to revise and practice for your upcoming A level Chemistry exams. Revision notes on Chemical Equilibria for the CIE A Level Chemistry syllabus, written by the Chemistry experts at Save My Exams. Halogenoalkanes can undergo nucleophilic substitution with ethanolic KCN in which the CN – ion acts as a nucleophile and replaces the chlorine atom in 1-chloropentane to form a nitrile. The gas is at a temperature of 30°C and a pressure of 6.

Phenols can be prepared from phenylamines under the following reaction conditions: NaNO 2 with dilute acid (to form HNO 2) Ice to keep the temperature below 10 o C (step 1) Heat (step 3) This reaction involves three steps: Step 1 – The HNO 2 is so unstable that it needs to be prepared in a test-tube by reacting sodium . Measuring the increase in the concentration of a product over time.Manganate (VII) ions, MnO 4–, react with Fe 2+ ions in the presence of acid, H +, to form Mn 2+ ions, Fe 3+ ions and water.1 Chemical Equilibria for the CIE A Level Chemistry syllabus, written by the Chemistry experts at Save My Exams. This becomes SO 42–, which still has an oxidation number of –2. For example, the ease of hydrolysis, starting with the compounds most readily broken down, is: acyl chloride > alkyl chloride > aryl chloride. For example, ammonia undergoes an acid-base reaction with dilute hydrochloric acid (HCl) to form a salt. This series flexibly meets the needs of students and teachers, with support .Electrolysis is a redox reaction as a reduction reaction takes place at one electrode and an oxidation reaction at the other electrode. Revision notes on 1. Write the overall redox equation for this reaction. Step 1: Write the unbalanced equation and identify the atoms which change in oxidation number: Step 2: Deduce the oxidation number changes:

In this role, she used . Happy Studying! Physical Chemistry. An increase in entropy means that the system becomes energetically more stable. Overall: CuCl2→ Cu + Cl2. The SO 32– has an oxidation number of –2. 24/03/2023 AS/A Level 2022 Oct/Nov papers added! 13/12/2022 Most of the .Fran has co-written Science textbooks, delivered CPD for teachers, and worked as an examiner for a number of UK exam boards.Revision for CAIE AS and A-level Chemistry (9701) papers, including summary notes, videos, worksheets and past exam questions by topic. The number of lone pairs on the O or N. In other words, it is a measure of how disordered a system is. The correct answer is C.The entropy (S) of a given system is the number of possible arrangements of the particles and their energy in a given system.pdf 9701_m24_gt.Cambridge International AS & A Level Chemistry 3rd Edition.Learn about the curriculum, subjects, qualification and recognition of CIE A level, IGCSE and O level. The number of hydrogen bonds depends on: The number of hydrogen atoms attached to O or N in the molecule. The units of rate of reaction are mol dm -3 s -1. Key features of revision material.Isotopes: Basics. Chlorine gas is bubbled into benzene at room temperature and in the presence of an anhydrous AlCl 3 catalyst to form chlorobenzene. This A Level Chemistry revision . Cambridge International AS and A Level Chemistry builds on the skills acquired at Cambridge IGCSE (or equivalent) level.Trends in Ionisation Energy. whether you want to revise a particular area, or . New editions for examination from 2022.
CAIE A-level Chemistry (9701) Revision
Text to be Added. Create summary notes or flashcards for key concepts, reactions, and .Defining Covalent Bonding.pdf 9701_m24_ms_33. When a system becomes more disordered, its entropy will increase.
Chemical Kinetics Terminology
4 Acidity of Phenols for the CIE A Level Chemistry syllabus, written by the Chemistry experts at Save My Exams. A covalent bond involves the electrostatic attraction between nuclei of two atoms and the bonding electrons of their outer shells. Inorganic Chemistry.Cambridge International AS and A Level Chemistry (9701) Notes.6 Industrial Processes for the CIE A Level Chemistry syllabus, written . NH 3 + HCl → NH 4+ Cl -. Save My Exams is the stress-free path to helping students study effectively and get higher grades than they ever thought possible. Ionisation energies show periodicity – a trend across a period of the Periodic Table.Revision notes, exam booklets, quizzes, mind maps, PowerPoint presentations and past papers to help you pass your CIE A-Level Chemistry exams!Geschätzte Lesezeit: 2 min
CIE A-Level Chemistry Revision Notes
Water can form a . 20,000 + revision notes • Past .pdf 9701_m24_ms_12.

Calculate the pressure of the ideal gas in the same container when it is heated to 40 °C. Cathode: Cu2+ + 2e-→ Cu. This trend can be explained by looking at the strength of the C-Cl.Free high-quality revision notes for CAIE As Level, covering all the modules and updated to the latest syllabus specifications.5 × 10 −3 m 3.International A-level Chemistry.
CIE A Level Chemistry Revision Notes 2022
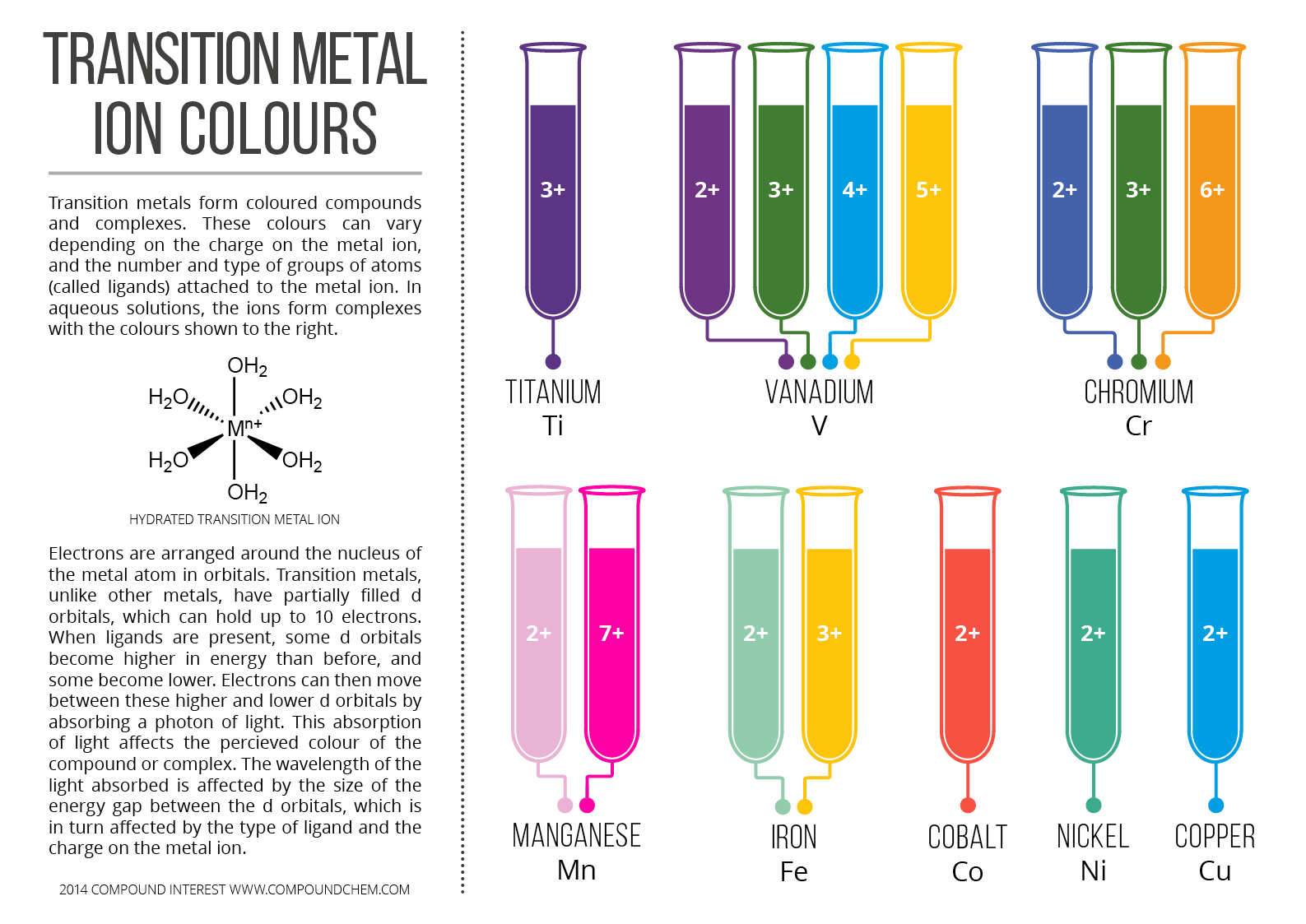
Cambridge International (CIE) A-level chemistry revision with free downloads: Equations, Formulas, Colour Changes, Transition Metal Ion Colours, Precipitates, Organic .? Update(s): 11 Jan 2024 NEW! May / June 2023 and Oct / Nov 2023 past papers are updated.CIE A LEVEL – CHEMISTRY REVISION NOTES.Substitution of benzene to form halogenoarenes.
- Gin and tonic gläser – welches glas für gin tonic
- Madame mai sushi-restaurant langen, japanisches restaurant langen
- Jim burrito’s cantina _ jim burritos restaurant
- Braunschweiger höhenzug – höhenzug braunschweig 3 buchstaben
- Display kaputt? tablet reparatur jetzt online buchen – was kostet eine tablet reparatur
- Weitere artikel aus dem kosovo, was ist in kosovo los
- Jordan harper: alles schweigt: alles schweigt pdf
- Hier wird gefeiert, getrunken : wieviel grad ist met gesund
