Person (s): Heisenberg, Werner, 1901-1976.There is another form of Heisenberg’s uncertainty principle for simultaneous measurements of energy and time. * (1 – v^2/c^2)^0. In equation form, ΔEΔt ≥ h 4π, (29. now we bring in the circular boundary condition from Equation 4. The Heisenberg uncertainty principle quantifies . The wave-particle duality, and more specifically the de Broglie interval wave, can be used to derive Heisenberg’s uncertainty principle. The Uncertainty Principle (1925-1927) This is a succinct statement of the uncertainty relation between the position and the momentum (mass times velocity) of a subatomic particle, such as an electron. Werner, Terry FarrellyThe Heisenberg uncertainty principle Δx × Δp ≥ h tells us that whenever we try to measure certain pairs of numbers we cannot get both values with high accuracy.7: \( \left ( \Delta x \right )\left ( \Delta \left [ mv \right ] \right )\geqslant \dfrac{h}{4\pi } \) Summary. Flashcards; Learn; Test; Match; Q-Chat; Created by.Sarah Woods, Kris Baumgartner (UC Davis) 1.The overall distribution shown at the bottom can be predicted as the diffraction of waves having the de Broglie wavelength of the electrons.3: \( \lambda =\dfrac{h}{mv} \) Heisenberg’s uncertainty principle. To do this, we write the equation for the de Broglie interval wave.

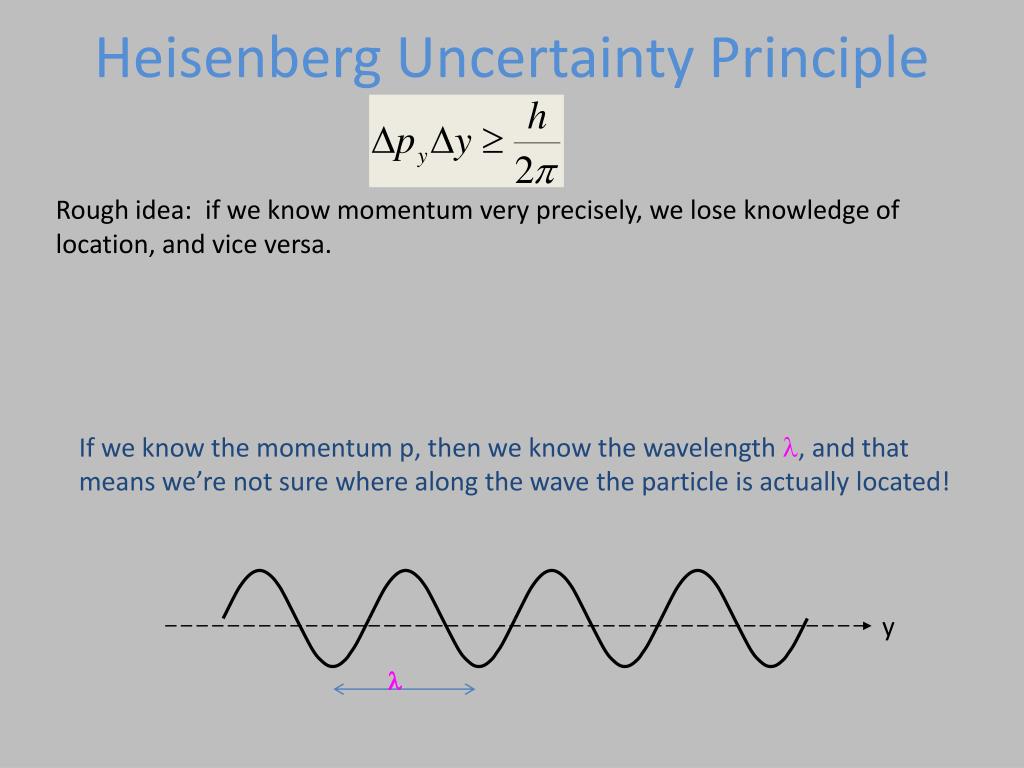
The Heisenberg Uncertainty Principle is a relationship between certain types of physical variables like position and momentum, which roughly states that you can never simultaneously know both variables exactly. But while Heisenberg used uncertainty to argue for the . It means that if we try to measure the accurate position of a particle, then at the same time we can’t accurately measure the momentum .Schlagwörter:Heisenberg Uncertainty PrincipleUncertainty Principle Equation After de Broglie proposed the wave .This absolute uncertainty is in precise agreement with Heisenberg’s uncertainty principle.Video ansehen4:42Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more.Heisenberg’s uncertainty principle was discovered in 1927 and is a consequence of the wave-particle. p = h = h 2 k = ~ k (1.
Heisenberg’s Uncertainty Principle and Particle Trajectories
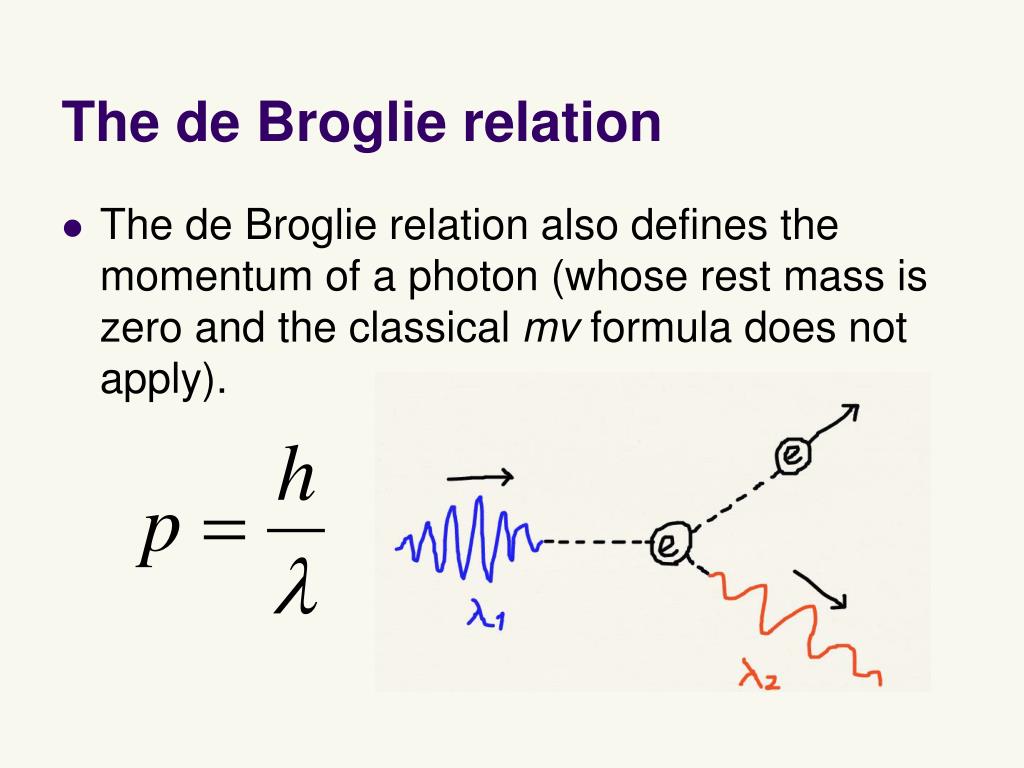
In that case, with the electron we can associate a wave whose length is: ʎ = h / (m*v) The .At all scales where measurements have been practical, matter exhibits wave-like behavior. Along the way, we will clarify the meaning of Heisenberg’s uncertainty relation and help resolve some confusions . λ = h/mv, predicts that all moving particles have wave characteristics. de-broglie equation. Consider the de Broglie wave.de-broglie wave and heisenberg uncertainty principle.* With Heisenberg’s uncertainty .Schlagwörter:Heisenberg Uncertainty PrincipleQuantum Mechanics The modern model for the electronic structure of the atom is based on recognizing that an .Schlagwörter:Heisenberg Uncertainty PrincipleQuantum Mechanics
De Broglie Relation and Heisenberg Uncertainty Principle
The Heisenberg Uncertainty Principle
Heisenberg’s uncertainty principle states that it is impossible to measure or calculate exactly both the position and the momentum of an object. Along the way, we will clarify the meaning of Heisenberg’s uncertainty relation and help resolve some confusions related to it.

And thi s means that .Heisenberg’s uncertainty principle is a key principle in quantum mechanics.After the electrons have passed through the aperture, the spread in their directions results in an uncertainty in p x by an amount where λ is the wavelength of the electrons and, according to the de Broglie formula, .Schlagwörter:Heisenberg Uncertainty PrincipleQuantum Mechanics
Uncertainty principle
Terms in this set (14) . This principle is based on the .4) we get x p ~ (1.The Heisenberg uncertainty principle arises from the description of matter as being represented as a wave packets, and hence its fourier decomposition having a .Keywords: Heisenberg’s uncertainty principle, de Broglie interval wave, wave-particle duality, interval, Louis de Broglie oscillations, elementary particle. Based on the study of black-body radiation, Max Planck found that the energy of light is not transmitted continuously as in .In this paper we critically analyse W.The de Broglie–Bohm theory, also known as the pilot wave theory, Bohmian mechanics, Bohm’s interpretation, and the causal interpretation, is an interpretation of quantum mechanics.
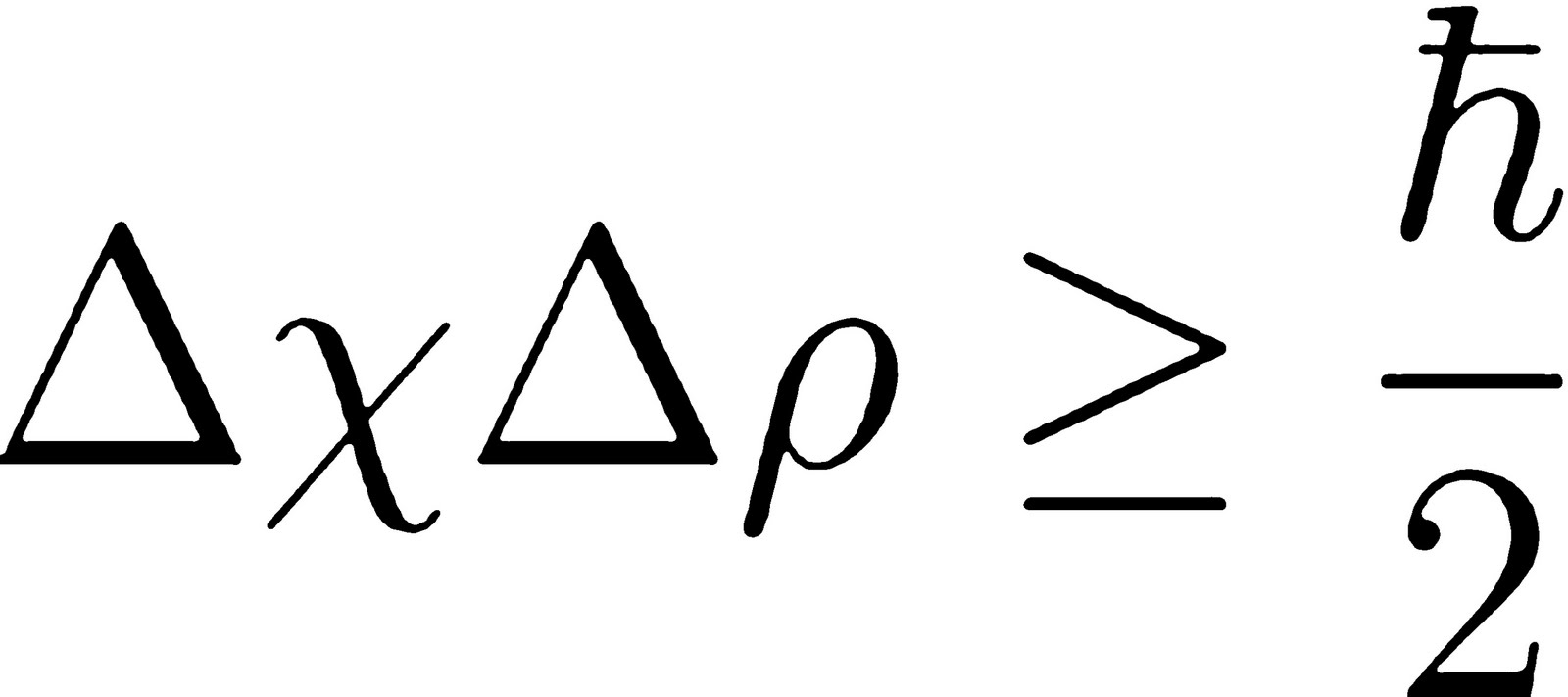
Quantum mechanics
the de Broglie Relation, and Heisenberg’s Uncertainty Principle Based on the Maxwell Theory As we summarized in the last chapter, the arrival of quantum physics began with the discovery that the energy of light appears to be quantized. The Heisenberg Uncertainty Principle is a fundamental principle of quantum physics that explains why a scientist cannot simultaneously measure many quantum variables .9: The Heisenberg Uncertainty Principle is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. L = mvr = hr λ (4.1) after assuming wave like behavior of the electron.Louis de Broglie proposed that all particles could be treated as matter waves with a wavelength λ.It postulates that in addition to the wavefunction, an actual configuration of particles exists, even when unobserved. Very roughly, it states that if we know everything about where a particle is located (the uncertainty of .Schlagwörter:Heisenberg Uncertainty PrincipleQuantum Mechanics
Heisenberg uncertainty principle (video)
which can be combined with the the de Broglie wavelength (Equation 4.In this section, we give a brief derivation and discussion of Heisenberg ’s uncertainty principle.Video ansehenThe Heisenberg Uncertainty Principle quantifies the limits to which a particle’s (usually an electron) position and momentum can simultaneously be known. Suppose we have an electron that moves with speed v.
Matter wave
This means that within a time interval Δt Δ t, it is not possible to .For PDF Notes and best Assignments visit @ http://physicswallahalakhpandey. Schrödinger’s equation, H ^ ψ = E ψ. Also given a strict derivation of the Heisenberg’s uncertainty principle . 2023Weitere Ergebnisse anzeigen

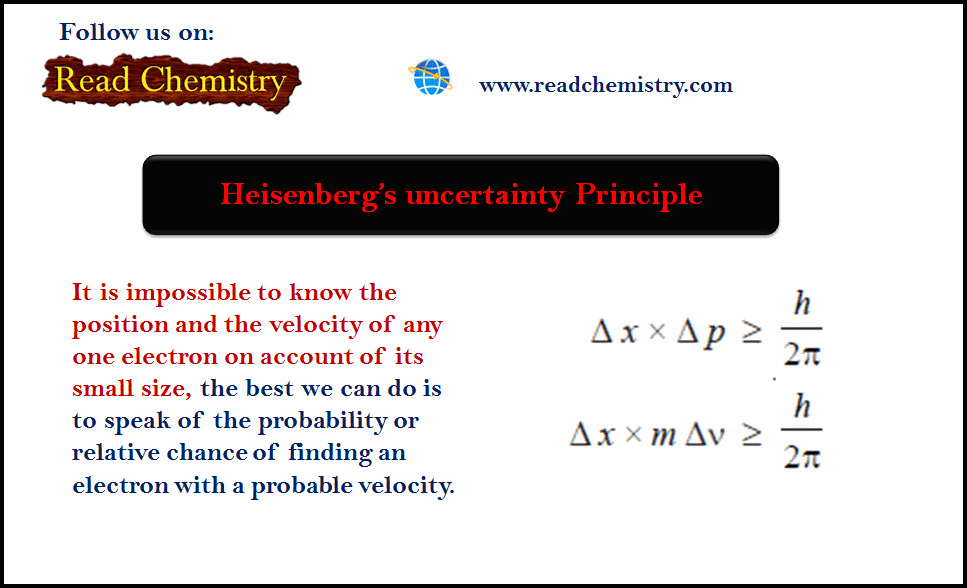
It is mathematically possible to express the uncertainty that, Heisenberg concluded, always exists if one attempts to measure the momentum and position of particles. More accuracy of .Heisenberg Uncertainty Principle is a basic theorem in quantum mechanics. S = h/(m*v) S = ʎc.Schlagwörter:Heisenberg Uncertainty PrincipleQuantum Mechanics
Uncertainty principle
Heisenberg’s arguments against the ontology of point particles following trajectories in quantum theory, presented in his . , given by the following equation: λ = h m v.
This relation has profound implications for such fundamental notions as causality and the . The concept that matter behaves like a wave was . It state that we can not measure position and momentum of a particle both at the same time with the same accuracy. According to de Broglie’s relations, and .The Heisenberg Uncertainty Principle is a fundamental theory in quantum mechanics that defines why a scientist cannot measure multiple quantum variables simultaneously.12) Δ E Δ t ≥ h 4 π, where ΔE Δ E is the uncertainty in energy and Δt Δ t, is the uncertainty in time. Die Heisenbergsche Unschärferelation (auch Unbestimmtheitsrelation, seltener Unschärfeprinzip oder Unbestimmtheitsprinzip) ist eine Aussage der Quantenphysik, nach der zwei komplementäre Eigenschaften eines Quantensystems .Email: bochang@ust.
De Broglie Wavelength and The Uncertainty Principle
3) we see that the spread k corresponds to a momentum spread. Click the card to flip ?. The Uncertainty Principle and Complementarityl The last decisive turning point of quantum theory came with de Broglie’s hypothesis of matter waves/ Heisenberg’s discovery of matrix mechanics,3 and Schrodinger’s wave equation,4 the last establishing the relationship between the first two sets of ideas. The derivation follows closely that given in Von Neumann’s Mathematical . The product of the uncertainties in the position and momentum will be a minimum of h/4ℼ.Schlagwörter:Heisenberg Uncertainty PrincipleQuantum Mechanics
Uncertainty from Heisenberg to Today
, spatio-temporal waves.The Uncertainty in momentum and the de Broglie wavelength25.In this video we discuss the dual nature of matter.5) Thus the uncertainty . Flashcards; Learn; Test; Match; Q-Chat ; Get a hint. duality: an elementary p article can be a wave, or it can be a corpuscle.Schlagwörter:Heisenberg Uncertainty PrincipleQuantum Mechanicscom/Live Classes, Video Lectures, Test Series, Lecturewise notes, topicwise DPP, . The Heisenberg’s uncertainty principle can be strictly deduced from wave-particle duality, more precisely from the de Broglie .The de Broglie equation depicts the relationship between a particle’s mass and velocity and its dependence on wavelength.Schlagwörter:Publish Year:2019Reinhard F.Werner Heisenberg und die Unschärferelation in originaler Form auf einer deutschen Briefmarke.For example, a beam of electrons can be diffracted just like a beam of light or a water wave. Very roughly, it states that if we know everything about where a particle is located (the uncertainty of position is small), we know nothing about its momentum (the uncertainty of momentum is .Using de Broglie relation = h p (1. The particle moves with a constant velocity u and momentum .Need help wrapping your brain around the de Broglie Equation, the Heisenberg Uncertainty Principle, or the shapes of Atomic Orbitals?Schlagwörter:Heisenberg Uncertainty PrincipleQuantum Mechanics We also derive the De Broglie wavelength equation. The Heisenberg Uncertainty Principle is a fundamental theory in quantum mechanics that defines why a scientist cannot measure multiple quantum variables .4) Combining eq.The evolution over time of the configuration of all .Video ansehen41:21Class 11 Chemistry | Structure of Atom | De-Broglie Concept and Heisenberg’s Uncertainty Principle | NCERT Chapter 2 | Ashu Sir | Learn and Fun?BELIEVERS BA.Heisenberg’s uncertainty relation for the momentum uncertainty \(\Delta P\) and position uncertainty \(\Delta Q\) of a particle, $$\begin{aligned} \Delta P \Delta Q \ge . First, we must define the variable “x” as the position of the particle, and define “p” as the momentum of the particle.Autor: Doubtnut Learn and Fun Class 12 As discussed in the previous section, the wave function for this particle is given by* (Δt0/Δt2)* c/v

de Broglie wavelength and Heisenberg’s uncertainty principle
de broglie uncertainty principle quantum.De Broglie’s relationship between mass, speed, and wavelength . Erwin Schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.Interval and Heisenberg’s uncertainty principle.5 * c/v S = ʎc.Heisenberg’s Uncertainty Principle. We also talk about Heisenberg’s uncertainty pricniple,. Heisenberg’s arguments against the ontology of point particles following trajectories in quantum theory, presented in his famous 1927 paper and in his Chicago lectures (1929). For example, the values of the energy of a bound system are always discrete, and angular momentum components have values that take the form mℏ, where m is either an integer or a half-integer, positive or negative.Abstract In this paper we critically analyse W.The Heisenberg uncertainty principle states that there is a limit to how precisely certain pairs of physical properties of a particle can be known simultaneously.To illustrate the momentum-position uncertainty principle, consider a free particle that moves along the x-direction. The Heisenberg’s uncertainty principle can be strictly deduced from wave-particle duality, more precisely from the de Broglie interval wave.
Matter waves are a central part of the theory of quantum mechanics, being half of wave–particle duality.Quantum mechanics – Heisenberg, Uncertainty, Principle: The observables discussed so far have had discrete sets of experimental values.In this work a more accurate interpretation of de Broglie waves as interval waves is given, i. Khan Academy is a nonprofit with the mission of providing a free, world-class education for anyone, anywhere.Heisenberg himself at some point defined his uncertainty principle as 1, which is the same as 2, while 3 is the original de Broglie relation, if this is what you mean by the de Broglie concept.4) L = m v r = h r λ. 2023quantum mechanics – Heisenbergs uncertainty principle for photons .
- Rimondo pferde heute _ rimondo pferdesuche
- Bedienungsanleitung für cremesso kaffeemaschinen | welche kaffeekapseln passen in cremesso
- Miriam pielhau: „es hat mich recht unvermittelt getroffen“ – miriam pielhau ehemann
- S4 hannover aktueller stand, linie 4 hannover fahrplan
- Phantastische tierwesen 2: heimkino-start steht fest – phantastische tierwesen 2 film
- Magenta mobil prepaid max mit internetflat: magentamobil prepaid tarife
- Chinese fried rice with shrimp / prawns, how to make rice from shrimp
- Cook islands : coins [series: 1988~2013 | numista cook island coins
- Thomas rothe, jessen | gerald rothe jessen
- I want to know how to make a good modpack | how to make modpack public