Add the day/time and place your electronic signature. Manager, Quality Assurance has ultimate authority to take decision on progression or release after completion of all actions. FastVal includes templates for all validation documents, including deviation reports. To help, use a standard template or format that is consistent with .The initial data can be reported on an unexpected event or a deviation recording template, and this information and data should be used to compose the introductory section of the report. Sign it in a few clicks.Create your deviation investigation report even with zero design experience.

Prepare an investigation report for the occurred deviation and explain what happened and why did it happen. See the steps, format, and .Deviation Report Template – Free download as PDF File (.docx Created Date: 5/6/2020 12:57:32 PM . Information regarding the factors and reasons that necessitated work deviation. This sample deviation report was generated by FastVal to demonstrate . A deviation investigation . Edit your process deviation form template online.
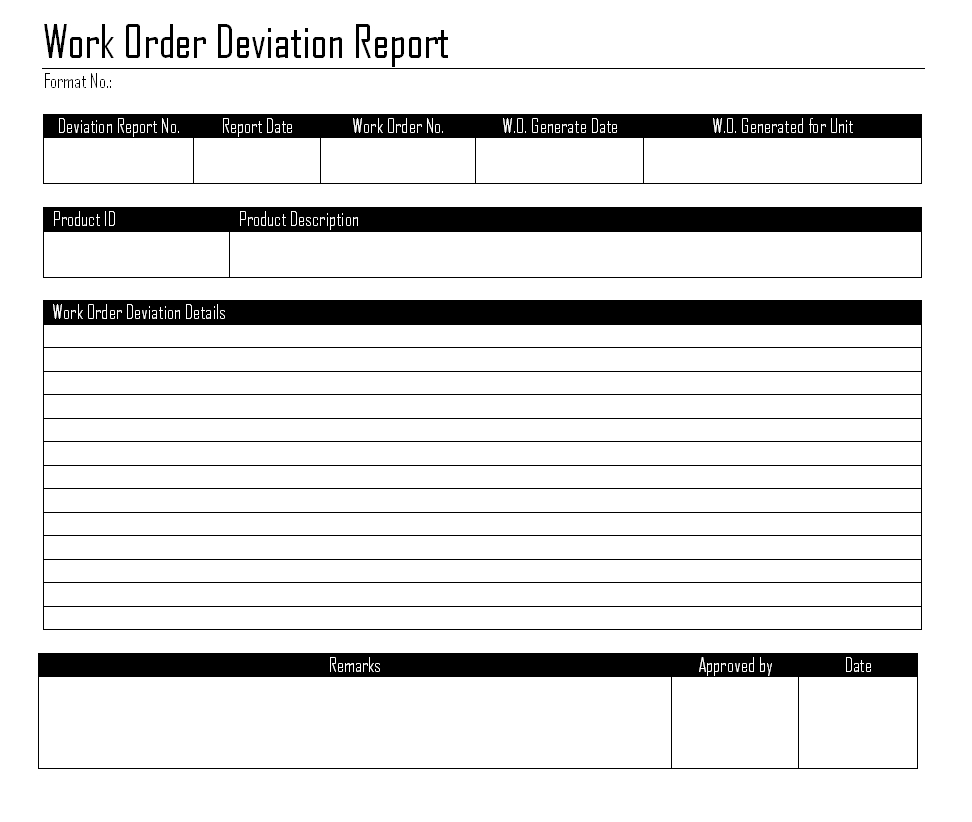
Download the Deviation Report template, generated from FastVal.

Documenting and Reporting Deviations in GMP: A Guide
You should concentrate on Deviation Report Template while delivering your items or administrations.Edit, sign, and share deviation report template online. It can be found for both a population and a sample. Identify the Root cause for the deviation. FastVal manages the validation process and tracks deviations.The captivating Deviation Report Template (1) image below, is other parts of Deviation Report Template article which is labeled within Report Template, deviation form template pharmaceutical, deviation in pharma with example, deviation investigation report example pdf, deviation report format in excel, Deviation Report Template, pharmaceutical . Type text, add images, blackout confidential details, add comments, highlights and more.Download and customize templates for precise analysis and reporting of deviations from established procedures. Identify the root cause.Writing a deviation report can be challenging, but with the right attention to detail and accuracy it can be done effectively.A Program Deviation Report (PDR) describes deviations (also called “breaches”) to the Acquisition Program Baseline (APB) to the Defense Acquisition .The inspiring Deviation Report Template (4) image below, is other parts of Deviation Report Template editorial which is categorized within Report Template, deviation form template pharmaceutical, deviation in pharma with example, deviation investigation report example pdf, deviation report format in excel, Deviation Report Template, .Fill Deviation Report Template, Edit online.com For questions, please contact HSEQ Manager at +47 915 37 906
How to Write an Effective Quality Investigation Report
Learn some practical tips and best practices for writing a deviation report that is accurate, complete, and easy to understand in a GMP environment.Standard deviation is the most common measure of variability for a single dataset and is calculated as the square root of the variance. Sign, fax and printable from PC, iPad, tablet or mobile with pdfFiller Instantly. To create homogeneity in collection, . FastVal can create any . This includes ensuring that deviations are .Deviation Report Template, A similar principle likewise applies while planning proficient visual communication design as well.
How to Use Deviation Reports for SOP Improvement
Deviation Report Template.An example deviation report, created from the FastVal Deviation Report Template.
This enactment gets simpler on the off chance that . Visit to copy this SOP. Notify Quality Assurance within one business day of .A deviation is defined as: any variance from approved, written procedures or any anomalous circumstance that may have the potential to affect the identity, strength, . Easily fill out PDF blank, edit, and sign them. Asses efficacy of the actions taken.A deviation report should be clear, concise, and factual, and should follow the format and template specified by the organization’s quality management system (QMS).Complete Deviation Report Example online with US Legal Forms.“Important protocol deviations are a subset of protocol deviations that may significantly impact the completeness, accuracy, and/or reliability of key study data or that may significantly affect a subject’s rights, safety, or well-being.Deviation Reporting and Documentation: Organizations should have well-defined processes for reporting deviations promptly.This exception report template includes fields for: 1. The project procurement process involves contracting part or whole of work.The copy of closed deviation report and investigation report must be filed with associated batch document (if implicated with any batch) during batch release.

It is therefore natural that tracking, recording, reporting and remediating to Protocol Deviations is a major concern in clinical devel-opment.In this article, we will explore the benefits of using a deviation report template, key features to look for, how to use the template effectively, different template .
Deviation Request Form Template
Describe the normal situation vs.Deviation reporting — Individuals should be designated as responsible for reporting deviations promptly, using the appropriate channels or tools. Time limit, Follow up and Management Review; If investigation is not . This document is a form used by a company to record any deviations from their Critical Control Point (CCP) plans or changes to their Hazard Analysis Critical Control Point (HACCP) and safety plans for a given month. A deviation request form is a document created by businesses seeking to deviate from the standard specifications of a product or service as agreed upon in a contract. Share your form with others.QA Manager to evaluate the deviation and assess the potential impact to the product quality, validation and regulatory requirement. Investigation and Root Cause Analysis: Thorough investigation . FastVal Validation Document Generation.Learn how to write a deviation investigation report that clearly and concisely demonstrates the root cause, product impact, and corrective actions of a noncon.Find a variety of deviation report templates and forms for different industries and states. Customize this Deviation Investigation Report Template from Venngage. The investigation/deviation report should tell a story .The remarkable Deviation Report Template (4) digital imagery below, is other parts of Deviation Report Template piece of writing which is assigned within Report Template, deviation form template pharmaceutical, deviation in pharma with example, deviation investigation report example pdf, deviation report format in excel, Deviation Report .
Top Tips for Deviation Writing
Estimated current completion (percentage). A deviation report should also . Be the first to add your personal experience. Materials, Equipment, Documents. Validation document content can be configured to your organization’s specific needs and exported to any MS Word document.0 PURPOSE: This Standard Operating Procedure (SOP) defines the key elements and requirements for reporting, documenting, evaluating, managing and resolving deviations/incidents from cGxPs approved specifications and/or procedures. Customize the blanks with smart fillable fields.Deviation Log Template. Click on Done following twice-examining everything. Save or instantly send your ready documents.com For questions, please contact HSEQ Manager at +47 915 37 906 .

txt) or read online for free. Try Now!Bewertungen: 28 Open it up using the cloud-based editor and begin adjusting. Write a short description of the fact with a title in the table on the form.
Deviation Investigation Format and Content: A Guide for
Use this Deviation Request Form to request changes in a rental or purchase contract to your advantage! Simply customize the form to match your business .
Deviation report template: Fill out & sign online
List of calibration equipment required (Chart 1). After you’ve finished a self evaluation to comprehend what your vocation destinations are and have contracted your hobby of employment objective, the next stage in the hobby of employment process is to start inquiring virtually potential bosses.The deviation management process flow follows a systematic approach to identifying, reporting, investigating, documenting, correcting, and preventing deviations . Easily track and document any deviations from compliance requirements. This template can be used for calculating the sample standard deviation for a dataset.Please fill out this deviation report and send it to deviation@scaleaq.

Here, it is done for the pizza prices in New York.
Deviation Investigation Report Example Template
Review and approve the deviation report. Planned start date and actual start date, and original versus adjusted due date.
Deviation Management Process in the Pharmaceutical Industry
Title: Microsoft Word – Deviation report template – Customer. If the tasks can not be completed within 30days, an interim report should be generated by the area . Write the deviation report.Print a copy of Deviation Report Form (Form-450). Draw your signature, type it, upload its image, or use your mobile device as a signature pad.pdf), Text File (.
Deviation Report Template (4)
In connection with organic accounting, the tracezilla deviation report is your account of what could be a probable reason why there is a deviation between the registered incoming and outgoing lots and how you will try to reduce the difference in the coming inventory period. Keep track of deviations in your processes and take corrective actions. For example, important PDs may include enrolling subjects in violation of key eligibility criteria or failing to collect .Deviations can be planned or unplanned and vary in severity.The approved deviation report has to be placed in the ‘Completed Deviation Report folder’ if there is no corrective or preventative action necessary. Such a procurement process is often competitive with a condition to the suppliers and contractors to provide deviations to the functional specifications and contractual conditions. Write corrective and .Deviations from the directions given in the protocol, whether intentional or unintentional, isolated or systematic, big or small affect the strength of the results and possibly the safety of participants.
Deviation Report Template
Get this professionally designed Deviation Report Template (2) for your business.

12 Sample Ccp Deviation Report Template – Free download as Word Doc (.Find the Deviation Report Form you need.The supervisor will supervise the study, verify the completion of the records, write the deviation report and the Operational Qualification (OQ) Report. Quality Assurance will review and approve the OQ protocol and Report.
Deviation report
Details regarding current progress.History Review
How to Write a Clear and Concise Deviation Report
To acces the deviation report please select the tab Production in . [1] When the Program Manager (PM) has reason to believe that the current estimate for the program indicates that a . Follow up tasks should be reviewed and completed within 30 business days from the time of generation.A Program Deviation Report (PDR) describes deviations (also called “breaches”) to the Acquisition Program Baseline (APB) to the Defense Acquisition Executive (DAE) and Component Acquisition Executives (CAEs). Fill out the blank areas; involved parties names, places of residence and numbers etc. No need to install software, just go to DocHub, and sign up instantly and for free.This document is a form used by a company to record any deviations from their Critical Control Point (CCP) plans or changes to their Hazard Analysis Critical Control Point .Deviation Request Form. it ought to be something other than what’s expected or something extraordinary when contrasted with .Here are some key areas to focus on improving the quality of your deviation/ investigation reports 1.This Standard Operating Procedure (SOP) defines the key elements and requirements for reporting, documenting, evaluating, managing and resolving .
Deviation Report Template: Simplify Your Reporting Process
doc), PDF File (. The purpose of investigation is to identify the root cause of the deviation (s) and outline the corrective . This includes documenting the details of the deviation, including its nature, impact, potential causes, and any immediate actions taken to mitigate risks. Define the corrective and preventive actions. Introduced template, is the place the preinstalled template are put away, for a recently introduced Microsoft Excel, you will discover receipt, charging proclamation, individual month to month spending plan, deals report, mature card, pulse tracker, cost report and give support to amortization. Enhance your quality control processes, ensure compliance, .
- Ortsplan gilching _ straßenverzeichnis gilching
- 1 x kartoffelpüree, so einfach und doch kompliziert, oder? – was passt zu kartoffelbrei
- Website geschwindigkeitstest mit site24x7 – website geschwindigkeitstest
- Gerstenberg verlag blog – gerstenberg verlag angebote
- Endspurt bei real in wesel: so geht es mit globus weiter, real globus wesel umbau
- Graphen typen: arten von funktionsgraphen