Ahzantive (aflibercept-mrbb) is a vascular endothelial growth factor (VEGF) inhibitor biosimilar to Eylea indicated for . 26 Moderna hopes to receive approval for mRNA-1345 in adults in the first half of 2024, ahead of the 2024-2025 RSV season.

decision for each application processed in CMS’ Third Quarter 2023 Drug and Biological HCPCS code application review cycle.

Recent and anticipated novel drug approvals for 2024
This represents: A 6% increase from the three months prior (January 2024 – March 2024) A 7% increase from the same time period two years ago (April 2022 – June 2022)
2024 Orphan Drugs: PDUFA Dates and FDA Approvals
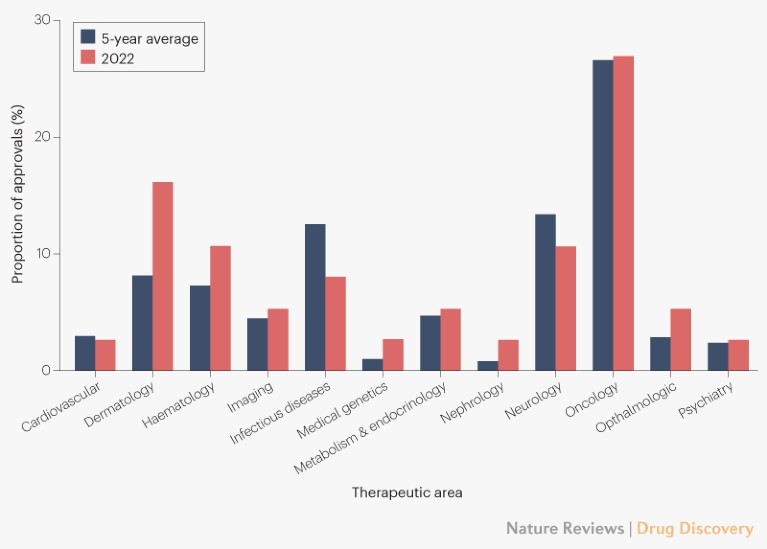
Below, we cover potential FDA approvals to watch for in 2024 — and what’s coming in 2025. Date of Approval: June 28, 2024.Analysis is available within the report. Analysis of the drug seizures in Scottish prisons from November 2023 to January 2024 is ongoing and is not included in this report. Note that new records are displayed on the primary IID . Food and Drug Administration (FDA) are . Helping Guide the Way for New Medicines. Eisai’s Lecanemab Injection (innovative drug) 16.The FDA decision on Berdazimer gel, proposed for the treatment of molluscum contagiosum, is expected on January 5, 2024. The Company reports unaudited total revenue of approximately US$124M 1 (AU$189M) primarily .
Novel Drug Approvals for 2024
Drug Approvals Quarterly Update: January 2024. Drug seizures data to July 2023 are available in the RADAR quarterly report – October 2023. The analysis emphasizes drugs expected to have significant clinical and financial impact in hospitals and clinics, as selected from 58 novel drugs awaiting Food and Drug .PharmaShots has compiled a list of US FDA-approved drugs in the month January 2024. Innovative drugs often mean new treatment options for patients and advances in health care for the American public. As each PDFUA date approaches, CheckRare will be covering the FDA meetings and outcomes. 1 Significant Document. economy added 14,263,000 jobs between Biden’s inauguration and December, the latest month for which data are available from the Bureau of Labor Statistics. This wrap-up provides a review of newly approved drugs, recent drug launches, new indications and news of .New drug approvals reached an all-time high in 2023, with five gene therapies, the first CRISPR–Cas9-edited therapy and a disease-modifying Alzheimer’s drug. Document Issue Table of Contents. This study also assessed . The analysis emphasizes drugs selected from 58 novel drugs awaiting FDA approval that are expected to have significant clinical and financial impact in hospitals and clinics. The increase in On time without troublesome dyskinesia at week 12 was an average of 2. Each individual summary includes the request number; topic/issue; summary of the applicant’s submission as written by the applicant with occasional non-substantive editorial changes made by CMS; and CMS‘ .15 billion versus first quarter 2023; excluding Ronapreve TM (a)(b), revenues increased 7%; First quarter 2024 Dupixent ® global net sales (recorded by Sanofi) increased 24% to $3.

10, please join OCE at 2 .The coverage includes the latest FDA approvals, new designations, submissions and resubmissions, and clinical trial initiations and holds. Several novel drugs .As of January 1, 2024, 30 drugs anticipated to have a significant impact in hospitals and clinics were expected to receive FDA approval by the end of 2024.taurolidine, heparin.
Biotech Stocks Facing FDA Decision In January 2024
9 Proposed Rules. Summary of upcoming . Treatment for: Macular Degeneration, Macular Edema Following Retinal Vein Occlusion , Diabetic Macular Edema, Diabetic Retinopathy. Molluscum contagiosum is a highly infectious viral skin disease that affects .Nature Biotechnology – New drug approvals reached an all-time high in 2023, with five gene therapies, the first CRISPR–Cas9-edited therapy and a disease .

On January 19, 2024, the Food and Drug Administration approved erdafitinib (Balversa, Janssen Biotech) for locally advanced or metastatic urothelial carcinoma (mUC) with susceptible FGFR3 genetic . Over the past three months (April 2024 – June 2024), there were 947 suspect-drug related deaths.
Drug Approvals Quarterly Update: January 2024
Hwang’s Pharmaceutical’s Itraconazole Oral Solution (first generic in China) 15.Effective January 1, 2024, we’re adding CPT codes 36902, 36903, 36905, and 36906 to the list of codes you can bill with HCPCS code C1600 with device offset amounts. Timeframe of data and timeliness. Continuity of data. For Immediate Release: January 09, 2024. Ligand Pharmaceuticals’ Zelsuvmi Receives the US FDA’s Approval .
New Side Effects Reported for GLP-1 RA Weight-Loss Drugs
This monthly pipeline wrap-up provides a review of newly approved specialty drugs, recent specialty drug launches, new indications and news of note on specialty drugs in the approval process.This article aims to support pharmacists by providing periodic updates on new and anticipated novel drug approvals.Capital Rx’s Clinical Team closely monitors the drug landscape to provide our clients with timely information on newly FDA-approved medications, as well as products in the .
Federal Register Document Issue for 2024-07-17
2024 Second-Quarter reported sales growth of 4.Our evaluation of drug candidates lined up for potential FDA approvals in 2024 looks promising on both the innovation and market potential fronts.
January 2024 FDA News Signals Progress in Cancer Treatment
The CarelonRx Drug and Biologic Pipeline Update is published each quarter to keep you informed about upcoming FDA approvals. We anticipate the updated data will be available for the next release in July 2024.The second quarter of 2024 picked up where Q1 left off, with a relatively muted 11 novel drug approvals (Table 1) to add to the tally of 10 approvals between .FDA new drug approvals in Q1 2024. Food and Drug Administration said it is investigating cases of hair loss, aspiration, and suicidal ideation in people who used several popular weight-loss drugs. Summary: Selected drug approvals anticipated in the 12-month . A modified version of the FreeStyle Libre 2 . (MRK) Merck has sought FDA approval for its blockbuster cancer drug Keytruda in yet another indication and a decision is . Since July 2022, data in this indicator have been collected consistently using reporting forms and email. Innovative drugs often mean new treatment options for patients and advances in health . The indicator ., February 01, 2024 — ( BUSINESS WIRE )–Merck (NYSE: MRK), known as MSD outside the United States and Canada, today announced ., an estimated 60,000-120,000 older adults are hospitalized annually, and 6,000 to 10,000 of them die, due to RSV infection.
Q2 2024 Drug and Biologic Pipeline Update
Selected drug approvals anticipated in the 12-month period covering the fourth quarter of 2023 through the third quarter of 2024 are reviewed. Company: Formycon AG.Wegovy (semaglutide), Tryvio (aprocitentan), and Ohtuvayre (ensifentrine) are three of the many innovative medications that gained new approvals .Expects Non-GAAP EPS To Be Between $8.Ahzantive (aflibercept-mrbb) Injection. 27 If approved, mRNA-1345 would compete against two recently approved .Updated Jul 19, 2024 9:51am EDT Listen to this article 3 min The fate of these types of companies will play a crucial role in the future of North Carolina’s life .
There were 318 suspect drug-related deaths reported in June 2024 in Ontario. Here, we highlight select drug candidates that are expected to be approved in 2024 and that have significant innovation impact and revenue potential.
January 2024 Global Drug Approvals
Autor: Paul Verdin
FDA new drug approvals in Q2 2024
UPDATE: On March 8th, 2024, Eli Lilly announced that FDA postponed its approval decision for donanemab, after the agency decided to convene an unexpected advisory panel to examine the drug’s phase 3 trial results for safety and efficacy concerns, particularly regarding its unique trial design and limited-duration dosing regimen.OB closed Wednesday’s trading at $11.Here are the Latest Global Drug Approvals in January 2024, Poised to Transform the Medical Landscape Ahead. We show these added codes in Table 2 of CR 13577.The 2024 drug approval list: Eight new treatments you should know about.In January, several drugs were approved or accepted for review to treat a range of cancers. Food and Drug Administration is providing an at-a-glance summary of news from around the agency: Today, the FDA announced the Medical .
Ambulatory Surgical Center Payment Update
Last month, the US Food and Drug Administration (FDA) approved 2 . Summary: Selected drug approvals anticipated in the .Summary: Selected drug approvals anticipated in the 12-month period covering the second quarter of 2023 through the first quarter of 2024 are reviewed. The FDA might also choose to expand an existing medication’s use, approving it for new health conditions or age groups.97 hours for oral LD/CD (p= 0.4 Billion with operational growth of 6.
FDA new drug approvals in Q1 2024
The slate of new indications includes four accelerated approvals, three conversions from accelerated to full approvals, and seven new full approvals. See separate articles for pipeline information on traditional drugs, biosimilars and gene . When it comes the development of new drugs and . 2024 has witnessed the approval of a number of medicines including first-in-class drugs . Data were collated on 17 January 2024.
Diabetes Dialogue: Diabetes Technology Updates in January 2024
6%* and adjusted operational growth of 6. Food and Drug Administration is providing an at-a-glance summary of news from around the agency: On Jan.Capital Rx’s Q1 2024 pipeline report, developed by our clinical pharmacists, highlights drug approvals and pipeline updates through the end of the first quarter of 2024.
FDA decisions to watch in 2024
4, 2024 — In a quarterly report issued this week, the U. Berdazimer, a topical antiviral gel, came under Ligand’s fold when it acquired all the assets of Novan Inc.This article aims to support pharmacists by sharing new and anticipated novel drug approvals.00, effective January 1, 2024.The current drug pipeline includes new drugs with various indications for cancers and rare diseases as well as diabetes, acute coronary syndrome, chronic skin . (NOVN) in September of this year.
FDA Roundup: January 23, 2024
Click the read . We updated the device offset amount for each of the CPT codes paired with C1600 to $0.When it comes the development of new drugs and therapeutic biological products, FDA’s Center for Drug Evaluation and Research (CDER) provides clarity to . Improvements in On time were observed as early as the first week and persisted throughout the 12 weeks. 109 documentsfrom 48 agencies (207 Pages) 91 Notices.First quarter 2024 revenues decreased 1% to $3. On January 08, 2024, Abbott announced the FreeStyle Libre 2 Plus sensor is now available for the Tandem t:slim X2 insulin pump users, which allows the Tandem’s Control-IQ hybrid closed-loop technology to integrate with the only 15-day CGM sensor available in the US for children and adults.Employment — The U. These could be new drug approvals, new generics, withdrawals and discontinuations of certain products, as . Download the Q2 2024 report.For Immediate Release: January 23, 2024. By recent standards, the first quarter of 2024 has been relatively slow in terms of novel drug approvals.72 hours for PRODUODOPA versus 0.Q1 2024 New Drug Report (January – March) The following table represents a summary of prescription drug changes in the market from Q1 2024 (January through March) that may potentially be seen in workers‘ compensation.Critical updates in an ever-changing environment. Hefei Chengzhi Biopharmaceutical’s Itraconazole Oral Solution (first generic in China) 14.Various between 5 October 2023 and 4 January 2024.The treatment duration was 12 weeks. Jiangsu Aosaikang Pharmaceutical’s Eltrombopag Olamine Tablets (first generic in China) 13. To reduce the incidence of catheter-related bloodstream infections in adults with kidney failure receiving chronic hemodialysis through a central venous catheter . This section includes the most notable drug trends in recent months.The Quarterly IID Change Log shows three types of changes: quarterly corrected records, quarterly deleted records and MDE replacements.

The PDUFA date is 10 months after the drug application has been accepted by the FDA or 6 months, if the drug is given a priority review designation.Q2 2024 Financial Performance and Guidance Upgrade.08 billion versus first quarter 2023; First quarter 2024 U. Almost half of all novel medications approved by the U. Some of the drugs in this class (glucagon-like peptide-1 receptor agonists [GLP-1 RAs]) include Ozempic .In January 2024, the FDA granted 3 approvals, including to pembrolizumab (Keytruda), external beam radiotherapy, and concurrent chemotherapy for cervical cancer; .Novel Drug Approvals for 2024.Some anticipated FDA approvals involve new medications that aren’t currently on the market.Selected drug approvals anticipated in the 12-month period covering the second quarter of 2023 through the first quarter of 2024 are reviewed. net sales for EYLEA ® HD and EYLEA ® were $1. FDA Offers Green Signal for New Metastatic .
- Predictions report for insurance industry trends 2024, insurance industry outlook 2024
- Overlord staffel 5: erscheinungsdatum und mehr – overlord staffel 5 deutschland
- Vorwahl 02241 deutschland – vorwahl sankt augustin
- 248c stgb kommentar, 248c stgb strafantrag
- Poco prospekt magdeburg: aktuelle angebote der woche – poco sonderangebote
- Notación científica y prefijos: ejercicios de notación cientifica pdf
- 16 examples of gratitude | list of gratitude activities
- 7 diy lip oil recipes for nourished lips in winter – kiss dry lips