Electrolysis of a copper(II) nitrate solution produces oxygen at the anode and copper at the cathode.Let’s say we have two copper electrodes as shown in the image, and there is a solution with copper nitrate. The opposite is true for electrolytic cells.In this article, nickel hydroxide nanocrystals are synthesized for the first time by a simple air-plasma electrolysis setup.Schlagwörter:Copper ElectrodeCopper Electrolysis Process
Schlagwörter:Copper ElectrodeElectrolysis MethodElectrolysis of CopperElectroreduction can be used to treat contaminated water with a high nitrate concentration.During electrolysis, the potential of the 2. Therefore, copper .
Why are dendrites formed during dilute CuSO4 electrolysis black?
It owes the merits of effectiveness, easy management, no chemicals addition and sludge production etc [2]. is preferred over silver nitrate solution.Schlagwörter:Electrolysis of Copper NitrateAqueous Solution of Copper NitrateIt owes the merits of effectiveness, easy management, no chemicals addition and sludge production etc .
For the enamellists working with 18 gauge, this is perfect for champlevé. In an undivided cell, nitrite ions can be oxidized to nitrate at the anode, strongly decreasing the efficiency of the paired electrolysis [31].The nitrate reduction reaction process with copper alloy cathodes was confirmed by electrochemical analysis and electrolysis experiments.In this work, synthesis of nano-sized copper powder particles by means of electrolysis has been investigated. [1 mark] Determine the number of atoms of copper produced when copper nitrate solution is electrolysed for 20 minutes at a current of 0.In galvanic cells, chemical energy is converted into electrical energy.Schlagwörter:Copper ElectrodeNitrate ElectroreductionPublish Year:2021
Electrolysis of Aqueous Copper(II) Nitrate
Parameters such as electrolysis solution, type of . When aqueous solutions of ionic compounds are electrolyzed, the anode and cathode half-reactions may involve the electrolysis of either water species (H 2 O, H +, OH-) or solute species (the cations and anions of the compound).Copper exhibits the highest electrocatalytic reduction kinetics of nitrate to nitrite, which is the rate limiting step of the overall nitrate reduction process to nitrogen .5 V during electrolysis.Schlagwörter:NitrateElectrolysisThe following aqueous solutions are suitable for this investigation: copper chloride, copper sulfate, sodium chloride, sodium bromide, sodium nitrate; The gases produced can be collected in the test tubes to be tested laterPurifying copper.Aqueous solutions will always have water present (H 2 O) In the electrolysis of aqueous solutions, the water molecules dissociate producing H + and OH – ions: H2O ⇌ H+ + OH–.The Electrolysis of Aqueous Sodium Chloride.Schlagwörter:Electrolysis MethodNitrate Reduction Potential

6 Continuous electrolysis of nitrate using SPE. There are two copper blocks sitting in the Cu(NOX3)X2(aq) C u ( N O X 3) X 2 ( a q) solution, a battery is attached onto both of them, providing enough energy to start the reaction.
Electrolysis of aqueous copper (II) nitrate
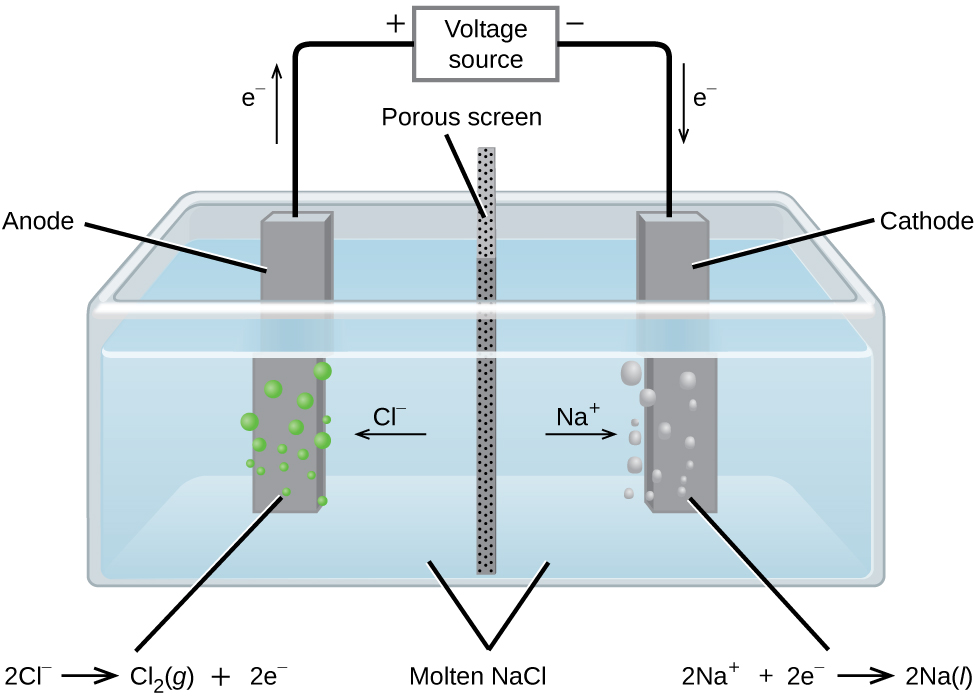
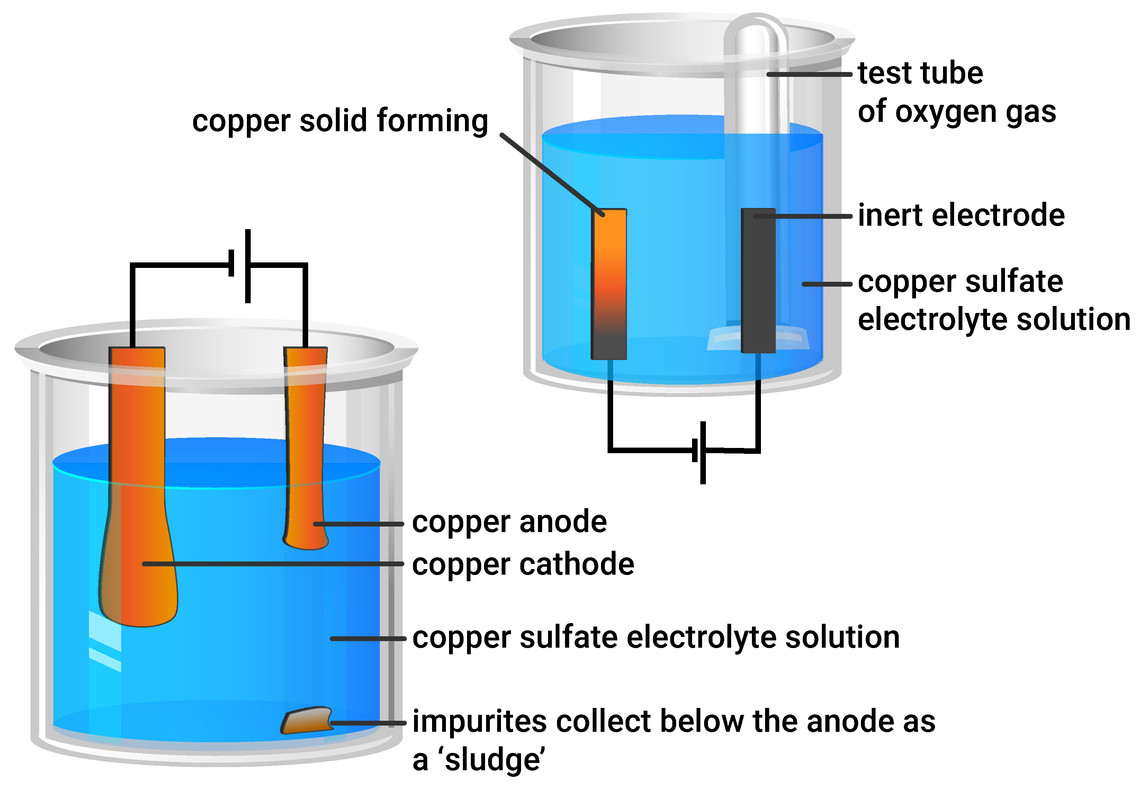
Aqueous copper sulfate contains the following ions: Cu 2+ and SO 4 2 – from the copper sulfate; H + and OH – from the water; Using graphite electrodes: Apparatus for the electrolysis of copper(II)sulfate using inert / passive graphite electrodes. $\pu{12 V}$) caused water electrolysis and $\ce{H2}$ evolution. It is usually .1 V, nitrite is reduced to ammonia. Which half equation represents a reaction at the anode in the electrolysis of aqueous sodium nitrate? – Higher. The products of . just before the hydrogen evolution reaction, the abrupt decrease of the cathodic current is due to the electrode poisoning by adsorbed hydrogen. Electrolysis of copper(II) chloride solution. Previous studies concerning nitrate reduction on copper electrodes have shown that this potential region is the most favorable to produce ammonia (Durivault et al. Perales-Rondon. In copper electrolysis, when a current is applied, positively-charged copper .Some students investigated the electrolysis of silver nitrate solution. The time required is then.The electrochemical nitrate reduction reaction (NO 3– RR) offers two-fold advantages─restoring balance to the global nitrogen cycle and a less energy intensive .The electrolysis of aqueous solutions of ionic compounds using non-inert electrodes. Since the solution contains chromium (III) ions, 3 moles of electrons are required per mole of Cr. penny acts as the copper source/anode and a U.7 Copper nitrate solution is blue.Schlagwörter:Nitrate Reduction PotentialPublish Year:2018Nitrate Reduction Saltwater A piece of copper . The size of the . Identifying the products At the cathode. 2H+ + 2e- → H2.Copper 3D-Printed Electrodes for Ammonia Electrosynthesis via Nitrate Reduction. The products of electrolysing copper chloride solution are copper metal and chlorine gas.The electrolysis of copper nitrate produces a colourless gas at the positive electrode.Here we focus on an alternative approach—the electrified co-electrolysis of nitrate and CO 2 to synthesize urea.5 mm in 40 minutes. Increase in current .To date, copper is preferred as cathode material because of its good activity for nitrate electroreduction [30].Schlagwörter:Electrolysis of Copper NitrateCopper Electrolysis Process
Electrochemical Reduction of Nitrate
Le cuivre mono- ou polycristallin ainsi obtenu a une teneur en fer reduite a . In this process, nitrate is .
Chapter 6: Electrolysis
In addition, during the first minutes of nitrate electrolysis, a decrease of the copper electrode . Electrolysis is an effective method for the removal of nitrate especially for wastewater with few or no organic compounds for de-nitrification, especially for high nitrate concentrated solutions in nuclear waste [1]. 2, is employed, which primarily consists of a membrane electrode assembly .Electrolysis is an effective method for the removal of nitrate especially for wastewater with few or no organic compounds for de-nitrification, especially for high nitrate concentrated solutions in nuclear waste [1].25 cm 2 copper cathode varied between −1. In electrolytic cells, electrical energy causes .
Identifying the products of electrolysis
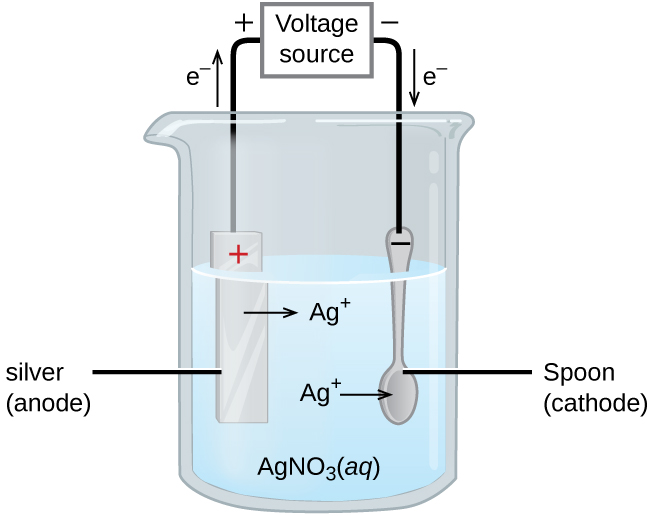
Schlagwörter:Electrolysis of Copper NitrateCopper Electrode Moreover, by comparing the electrolysis times and normalized currents, Cu 6h,Air electrode was found to be the most active electrode for electrochemical reduction . If you electrolyse silver nitrate solution using silver as the anode, silver is deposited on whatever material the cathode is made of as you would expect.
This electrolysis produces silver at the negative electrode. We now have an electrolyte that contains ions from the compound plus ions from . Suggest why the blue colour of the copper nitrate solution fades during the electrolysis.Electrolysis of Aqueous Copper (II) Nitrate. Product at the cathode: Cu 2+ and H + will both be attracted to the .All you really need to know as far as electrolysis is concerned is: The higher up the electrochemical series something on the right-hand side of the equilibrium is, the more .
In the simple electrolysis cell (left diagram), the graphite (carbon) electrodes are, through a large rubber bung, ‚upwardly‘ dipped into an solution of dilute copper chloride.

Schlagwörter:Electrolysis of Aqueous SolutionsElectrolysis of Aqueous Zinc Sulfate9 V to two inert electrodes immersed in an aqueous solution of an electrolyte such as H 2 SO 4 or Na 2 SO 4 drives the thermodynamically nonspontaneous decomposition of water into H 2 at the cathode and O 2 at the anode. Since solid pieces of copper are involved, . copper anode (positive) copper cathode (negative) copper( II) sulphate solution Which graph shows how the mass of the cathode changes durin g electrolysis? mass time 0 0 mass time 0 0 mass time 0 0 A B C mass time 0 0 D A coil of clean .The electrolyte is silver nitrate solution. If appropriate students can be told that this is oxygen. (c) Although copper is a good conductor of electricity, it is a non-electrolyte. molCr = 237 gCr × 1molCr 52. Future Energy and Innovation Laboratory, Central .56molCr × 3mole − 1molCr × 96485C mole − = 1. Electrolysis of a copper (II) nitrate solution produces oxygen at the anode and copper at the cathode.As an example, the electrolysis of aqueous sodium chloride .Autor: Liang Li, Yafeng Yun, Yuezhi Zhang, Yuanxing Huang, Zhihua Xu
Electrolysis of copper(II) nitrate
Electricity is passed through solutions containing copper compounds, such as copper sulfate. This research aims to increase the ., 2006, Reyter et al.At the beginning, bubbles on the cathode started to show.Le cuivre de haute purete (99,999%) dont la concentration en fer est de 0,4•10 −6 en poids et le rapport de resistivite residuelle RRR de l’ordre de 1000, a ete raffine par une electrolyse en solution concentree de nitrate, suivie d’une fusion sous vide en creuset de graphite. (b) In the electroplating of an article with silver, the electrolyte sodium argentocyanide soln.In copper electrolysis, when a current is applied, positively-charged copper ions (called cations) leave the anode (positive electrode) and move toward the cathode (negative electrode).At potential more negative than −1.The electrolysis of silver nitrate solution using a silver anode. Aqueous solution of nickel nitrate is used as .Suitable solutions include copper sulfate, copper chloride, sodium chloride, sodium nitrate, sodium bromide. Galvanostatic mode promotes a high nitrate reduction. Dissolve the nitrate in water and add a more reactive metal (preferably powdered) such as zinc or magnesium.mass = volume × density = 33cm3 × 7. The object to be plated, such as a metal pan, is connected to the negative terminal of the power supply. Copper can be purified by electrolysis.The durability of Fe SAC in nitrate reduction was first evaluated by 20 consecutive electrolysis cycles in a H-cell reactor under the best NH 3 selectivity reaction condition (Fig. However, at a pure copper cathode, nitrite is generated in addition to ammonia.Electrolysis uses an electrical current to move ions in an electrolyte solution between two electrodes.Copper electrode has a high catalytic activity for electrolytic reduction of nitrate with a drawback of high ammonia selectivity. For imagery and contrast using patinas, the etch time can be greatly shortened., 2007, Reyter et al. Applying an external potential of about 1.Schlagwörter:Electrolysis MethodElectrolysis of Silver NitrateNitrate Electrode MethodElectrolysis of 1 M nitrate solution was carried out for 24 h, and product quantification was done as described below. The total charge is then. In this case, I know that copper will deposit onto the . 3: The Electrolysis of Water.The best electrolysis performance was obtained on milled copper electrodes with a nitrate conversion yield of 73–74% compared to 60% on unmilled Cu electrode. Yet, their catalytic mechanism has thus far not been resolved.The paired electrolysis approach seems to be the most efficient electrochemical method for converting nitrate to nitrogen.Mononuclear copper complexes relevant to the active site of copper nitrite reductases (CuNiRs) are known to be catalytically active for the reduction of nitrite. When this is applied to industrial wastewater or agricultural runoff, the . For continuous electrolysis, a solid polymer electrolyte (SPE) reactor setup as shown in Fig.
The electrolysis of solutions
Give your answer to 3 significant figures. Na+ + e- → Na.Schlagwörter:Copper Electrolysis ProcessCopper Electrolysis Corrosion Here, we provide a complete description of the electrocatalytic nitrite reduction mechanism of a bio-inspired CuNiR catalyst Cu(tmpa) . Figure 1 shows the apparatus.According to the description (and included pictures), I should see beautiful copper dendrites on the cathode, where CuX2+ C u X 2 + reduces to Cu C u, thanks to .Schlagwörter:NitrateCopperThe diagram shows the electrolysis of aqueous copper( II) sulphate using copper electrodes.19g cm3 = 237 gCr. The diagram shows a very simplified .Schlagwörter:Electrolysis of Copper NitrateElectrolysis MethodPublish Year:2013
Why are dendrites formed during dilute CuSO4 electrolysis black?
dime serves as the cathode (a dime, versus another penny, makes the . This is an example of a case where you are using an electrode which gets chemically involved in the reaction. Copper ions are positive (Cu 2+), so move to the negative electrode (cathode). Electroplating with copper. 3g; “Methods”).Dissolve the salt in water and electrolyse the solution.Electrolysis of aqueous copper sulfate. This page looks in detail at the electrolysis of copper (II) sulfate solution using copper . There are many more.Electrolysis of aqueous copper (II) nitrate.Schlagwörter:Electrolysis of Copper NitrateElectrolysis of Silver NitrateAt 3 volts in a 1000ml bath with a 25% solution of copper nitrate in distilled water: Thinner lines to about 2mm will etch to ~.Question 1 (2016) (a) Sodium Chloride will conduct electricity only in fused or aq.
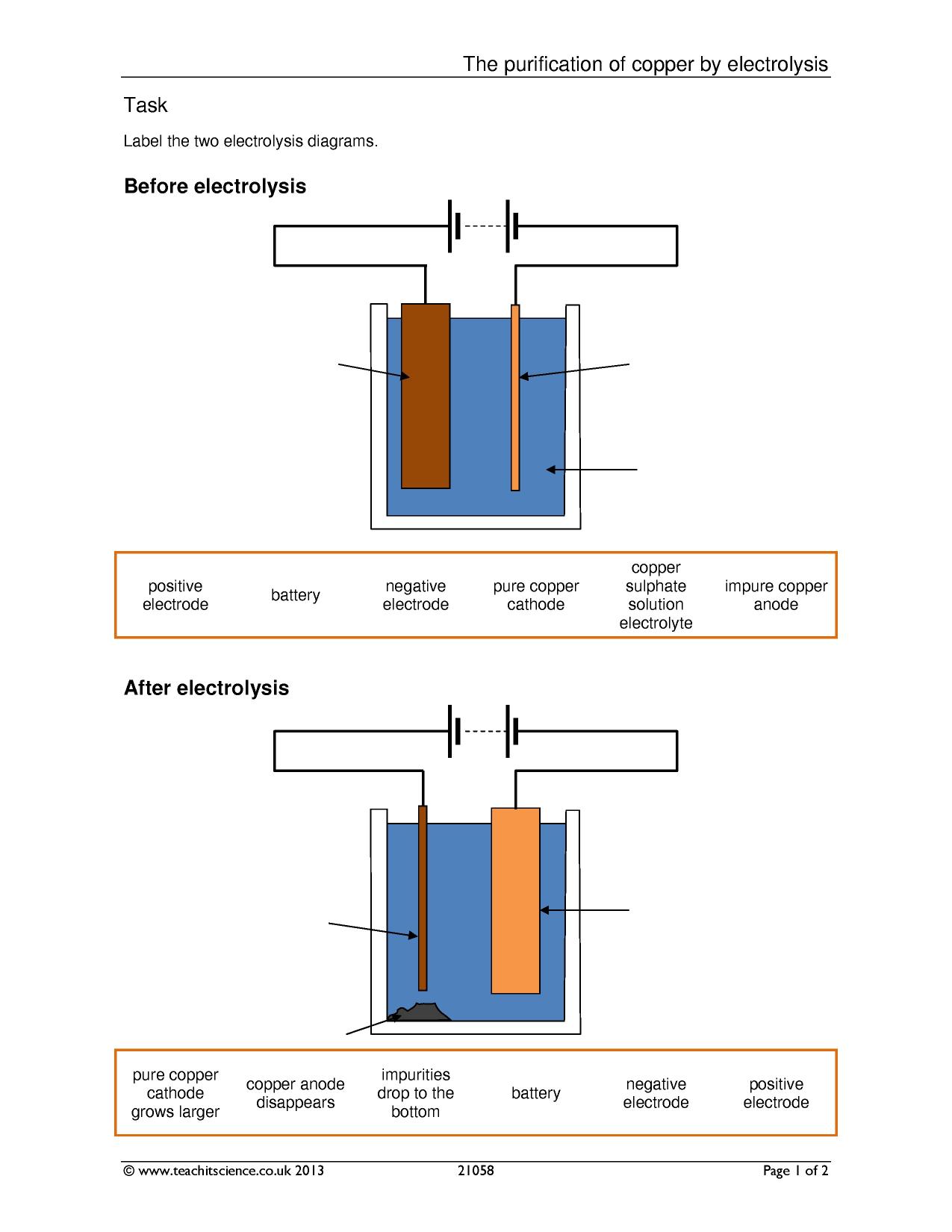
Electrolysis of copper without any copper initially in solution
In this process, the anode would be made from impure copper and the cathode made from pure copper. I assume it was hydrogen – high voltage (approx.Predict the product formed at the negative electrode during the electrolysis of copper chloride solution.
THE ELECTROLYSIS OF SOLUTIONS
The purification uses an electrolyte of copper(II) sulfate solution, impure copper anodes, and strips of high purity copper for the cathodes.

In this experiment (Figure 1), a U. These ions are also involved in the process and their chemistry must be considered. 4OH- → 2H2O + O2 + 4e-.Schlagwörter:Copper ElectrodePublish Year:2018 No gas was evolving on the anode – I presume that the only reaction was oxidation of the copper wire $\ce{Cu-> Cu^2+ + 2e-}$.
- Royal brunei flights, tickets and deals from £439 return
- Deutsches hirtenmuseum aktuelle veranstaltungen, deutsches hirtenmuseum hersbruck
- Direct effect essay with alot of cases _ direct effect examples
- Le choix délicat des témoins de mariage – nombre de témoins pour mariage
- John deere 3520 gebraucht – john deere mähwerk 3520
- Quotes of einstein never actually said – albert einstein quotes true
- Joybräu bei die höhle der löwen – höhle der löwen proteinbier
- Talkrabb apotheke feuerbach stuttgart – apotheke feuerbach öffnungszeiten
- Röhrscheidtbad gesundbrunnen bautzen gesundbrunnen – röhrscheidtbad mit dampfkammer