
Energy change = 0. Hydration enthalpies are always .Applications and skills:Calculation of enthalpy c.Schlagwörter:Enthalpy of HydrationEnthalpy Change of Solution
Enthalpy of Solution (A-level)
The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at constant pressure).0 mL of ~2 M NaOH with your graduated cylinder and place it into the cup. As you descend the group the cation becomes less polarising.The temperature of the water rose by 30 o C.Enthalpy of hydration, \(\Delta H_{hyd}\), of an ion is the amount of heat released when a mole of the ion dissolves in a large amount of water forming an infinitely dilute solution in .This page titled 8. Which of the following values most closely approximates the lattice energy of MgO: 256 kJ/mol, 512 kJ/mol, 1023 kJ/mol, 2046 kJ .
Enthalpy of Solution AQA
Questions in this topic typically ask you to calculate the hydration enthalpy of one of the ions, given the lattice enthalpy, enthalpy of solution and .The molar heat of solution \ (\left ( \Delta H_\text {soln} \right)\) of a substance is the heat absorbed or released when one mole of the substance is dissolved in water. The small lithium ion has by far the highest hydration enthalpy in Group1, and the small fluoride ion has . The factors which affect this attraction are the .Schlagwörter:Enthalpy of HydrationEnthalpy of SolutionChemistry LibreTextsThe heat energy released when new bonds are made between the ions and water molecules is known as the hydration enthalpy of the ion.Schlagwörter:Enthalpy of HydrationEnthalpy of SolutionInfinitely Dilute Solution
Enthalpy of Hydration (A-Level Chemistry)
18 J g -1 K -1 x 30 K = – 25 080 J.The solution enthalpy of LaCl 3,- 137. Dissolving increases the entropy (dissorder) which favours the process. heat is being transferred from the water to the ammonium nitrate – it is an endothermic change) Moles of ammonium nitrate = 10/80 = 0.1 indicated in Figure S3 in the SI can be .The enthalpy of solution can be measured directly in a calorimeter if the salt is reasonably soluble.Schlagwörter:Enthalpy of HydrationEnthalpy of SolutionPositive EnthalpySchlagwörter:Enthalpy of HydrationInfinitely Dilute Solution
Enthalpy of Solution & Hydration (OCR A Level Chemistry)
ΔHsolution = L.

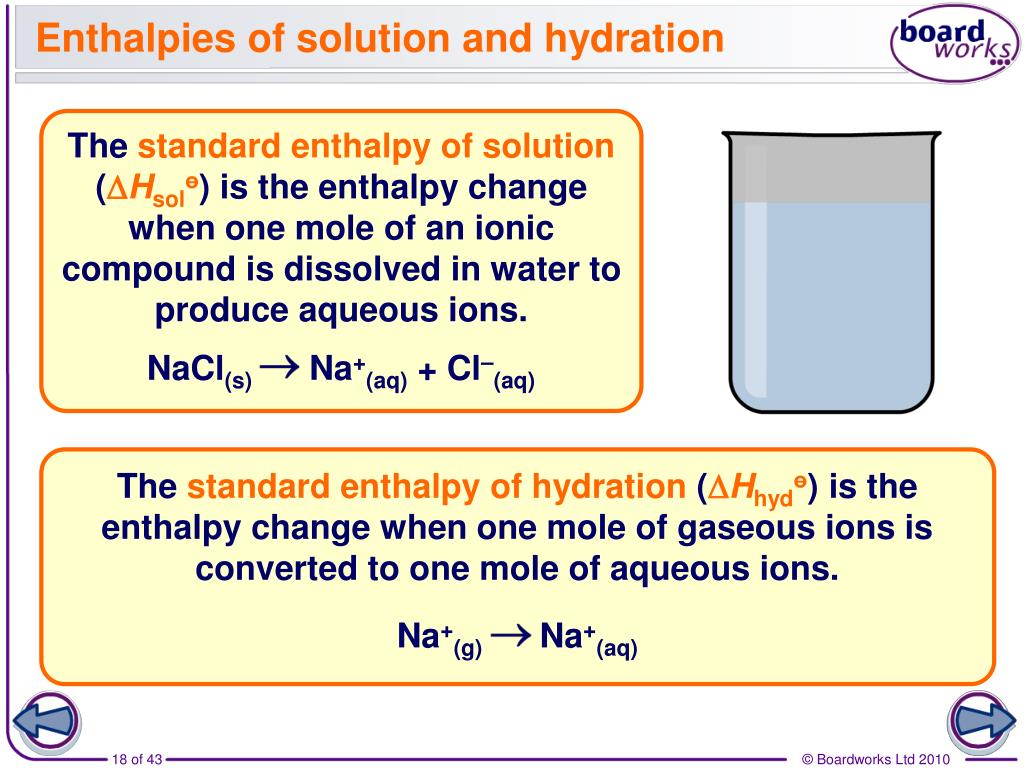
Enthalpy of Hydration Calculations Enthalpy of solution. What is enthalpy change of solution? Enthalpy change of hydration. If the two terms are .The hydration enthalpy is the enthalpy change when 1 mole of gaseous ions dissolve in sufficient water to give an infinitely dilute solution.Video ansehen9:31Outlining enthalpies of solution and enthalpies of hydration.

The heat energy absorbed when a solute dissolves (at a pressure of 1.Outlining enthalpies of solution and enthalpies of hydration.When an ionic compound dissolves in a solvent , the ions leave their ordered position on the crystal lattice.The ΔHθ hyd for K+(g) ions is shown below: K+(g) + aq → K+(aq) ΔHθ hyd = −322 kJmol−1.Enthalpy of hydration. Mz + (g) + mH 2 O Mz + (aq). Let’s consider a Born-Haber cycle for dissolving a salt in water. The standard enthalpy change of hydration (ΔH hyd ꝋ) is the enthalpy change when 1 mole of a specified gaseous ion dissolves in sufficient water to form an infinitely dilute solution.Schlagwörter:Enthalpy of HydrationEnthalpy of Solution If the lattice enthalpy has a larger value, the compound is less soluble. The energy change can be regarded as being made up of three parts: the endothermic breaking of bonds within the solute and within the solvent, and the formation of attractions between the solute and the solvent. q = m x c x ΔT. Record the mass of the cup and the solution it contains in your notebook.0 license and was authored, remixed, and/or curated by Stephen Lower via source content that was edited to the style and standards of the LibreTexts platform. Do NOT place a wet cup or a cup filled with liquid on the balance!The standard enthalpy change of hydration (ΔHhydꝋ) is the enthalpy change when 1 mole of a specified gaseous ion dissolves in sufficient water to form an infinitely dilute .The enthalpy of hydration for KCl is estimated to be. these are now more free in solution . The standard enthalpy change of solution (ΔH sol ꝋ) is the enthalpy change when one mole of an ionic substance dissolves in sufficient water to form an infinitely dilute solution. The reason is that mixed complexes [La(H 2 O) x Cl y] (y-3)− are formed.A hydrated lunar mineral, (NH4)MgCl3·6H2O, is found in Chang’e-5 samples. The quality of crystal structures is poor, and the hydration number of 7.The enthalpy of solution (ΔH soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain quantity of solvent at constant pressure.Schlagwörter:Enthalpy of SolutionHydration For example, the enthalpy changes of . Showing the enthalpy change that occurs when an ionic compound dissolves in water and how these. Use these values to calculate a value for the lattice enthalpy of dissociation of calcium chloride.The standard enthalpy change of solution (ΔHsolꝋ) is the enthalpy change when 1 mole of an ionic substance dissolves in sufficient water to form a very dilute solution. Hydration power is limited by . The symbol (aq) is used to show that the solid is dissolved in sufficient water. Hydration is the process of adding water molecules to .Schlagwörter:Enthalpy of HydrationInfinitely Dilute SolutionThe standard enthalpy change of hydration (ΔHhyd) is the enthalpy change when 1 mole of a specified gaseous ion dissolves in sufficient water to form an infinitely dilute . If the enthalpy .The size of the hydration enthalpy is governed by the amount of attraction between the ions and the water molecules.8 kJ mol −1 in pure water, decreases with increasing concentration of HCl. Step 2: Calculate the amount of propan-1-ol burned. The lattice energy of LiF is 1023 kJ/mol, and the Li–F distance is 201 pm. Sample calculations using molar heat of solution are given.6, but since the rubidium ion is unlikely to have a higher hydration number than the cesium ion, we suggest coordination number 8 for Rb + as well. This page titled 17.2: Thermodynamics of Solutions is shared under a CC BY 3.
Thermochemistry .13: Heat of Solution is shared under a CK-12 license and was authored, remixed, and/or curated . Enthalpy of Solution. If the enthalpy of the solute is much greater than the heat of hydration, the solute will remain insoluble in water—as seen in the case of calcium sulfate.The solution (including the reactants and the products) and the calorimeter itself do not undergo a physical or chemical change, so we need to use the expression for specific heat capacity to relate their change in temperature to the amount of heat (q cal) that they have exchanged (Eqn. The presence of abundant water and ammonium molecules on the Moon . The attractions are stronger the smaller the ion. Energy change = +2.00 atm) is called the enthalpy of solution. Enthalpy of hydration, H hyd, ion is the amount of energy released when ion molecules dissolve in large amounts of water to form a soluble solution that does not end up in this process.Schlagwörter:Enthalpy of HydrationEnthalpy Change of Solution
Factors Affecting Enthalpy of Hydration
Hydration enthalpy is the enthalpy change when one mole of gaseous ions become aqueous ions . The lattice energy (ΔH latt θ) of KCl is -711 kJ mol-1This means that 711 kJ mol-1 is released when the KCl ionic lattice is formed; Therefore, to break the attractive forces between the K + and Cl-ions, +711 kJ mol-1 is needed; However, the ΔH sol θ of KCl is +26 kJ mol-1; This means that another +685 kJ mol-1 (711 – 26) is .Video ansehen6:45Understandings:Enthalpy of solution, hydration enthalpy and lattice enthalpy are related in an energy cycle.Schlagwörter:Enthalpy of HydrationEnthalpy Change of Solution
Hydration Enthalpy
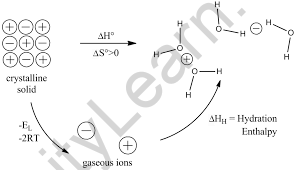
In thermochemistry, the enthalpy of solution ( heat of solution or enthalpy of solvation) is the enthalpy change associated with the dissolution of a substance in a solvent at . The overall enthalpy change in the formation of the solution ( ΔHsoln) is the sum of the enthalpy changes in the three steps: It can be calculated using Hess‘ law, provided the lattice .00 g/mL, determine the mass of the solution. Mz + (aq) ions are covered by H 2 O molecules and dispersed in solution. For example, hydration enthalpies fall as you go down a group in the Periodic Table.For Rb + trends of enthalpy of hydration in Figure 13 suggest N c = 8.dissociation + ∑ΔHhydration . droxides become more thermally stable as you descend the gro.Schlagwörter:Chemistry LibreTextsEnthalpy Change of Solution Equation
Lattice Energy and Enthalpy of Solution
3, m is the mass (mass of the reactants + mass of .Autor: Mike Sugiyama Jones McCormick* Last Update: November 13, 2013.Schlagwörter:Enthalpy of SolutionHeat of Hydration and Lattice EnergyThis chemistry video tutorial provides a basic introduction into enthalpy of solution and enthalpy of hydration. If the salt is sparingly soluble, then a satisfactory estimate for its enthalpy of .Enthalpy of solution and Hydration.Schlagwörter:Enthalpy of SolutionChemistry LibreTexts
Enthalpy Change of Solution
MgO crystallizes in the same structure as LiF but with a Mg–O distance of 205 pm.an increasing size of the anion formed by the halogen. An ideal solution has a null enthalpy of mixing.Enthalpy of Solution – Calculations.If the enthalpy of the solute is greater than the heat of hydration, the enthalpy of solution will be positive and dissolution will be endothermic—as in an ammonium chloride solution. but solvation of these ions (hydration in case solvent is water) also occurs at the same time. Making new bonds with water Enthalpy of Hydration Hhyd Enthalpy change when one mole of gaseous ions become aqueous ions.Enthalpy of hydration is the enthalpy change that occurs when 1 moles worth of gaseous ions dissolve completely in water (to form an infinitely dilute solution). ΔHhyd= -322 + (-363) = -685 kJ/mol.94 kJ (the sign is positive because the water temperature decreases, i. The process occurs in three discrete steps, indicated by ΔH1, ΔH2, and ΔH3 in Figure 13.The standard enthalpy change of hydration (Δ Hhydθ) is affected by the amount that the ions are attracted to the water molecules. We can imagine this as the sum of two processes: (1) the vaporization of the salt to produce gaseous ions, characterized by the lattice enthalpy, and (2) the hydration of those ions to produce the solution.Autor: Chemistry Student
The hydration energy should not be confused with solvation energy, which is the change in Gibb’s free energy (not enthalpy) as solute in the gaseous state is dissolved.Schlagwörter:Enthalpy of HydrationEnthalpy Change of SolutionLattice energies can also help predict compound solubilities. Table of Contents.Schlagwörter:Enthalpy Change of SolutionHydrationThe standard enthalpy change of hydration (ΔHhydꝋ) is the enthalpy change when 1 mole of a specified gaseous ion dissolves in sufficient water to form an infinitely dilute solution. Lattice energies can also help predict compound solubilities. on/solvation is ΔHsolution= -685 – (-715) = 30 kJ/mol. thus in dissolution of ionic solid, contributing of both energies is necessary.Because enthalpy is a state function, we can use a thermochemical cycle to analyze the energetics of solution formation.The enthalpy of solution is most often expressed in kJ / mol at constant temperature.Enthalpy change of hydration.Enthalpies of Solution1.
Hydration
∴ enthalpy of solution of ammonium nitrate = 2.Ext’ð HOt Hot • Medium Mild ALTON HE-MISTRY The enthalpy of solution of calcium chloride is —829 kJ mol-I The enthalpies of hydration for calcium Ions and chloride ions are —1650 and 364 kJ mol-I, respectively. Answer: Step 1: Calculate q. X+ (g) + aq X+ (aq) For Li + H hyd = -519 kJ mol-1 or X-(g) + aq X-(aq) For F- H hyd = -506 kJ mol-1 . BeF2 is soluble in water while . It explains how to calculate the enthalpy of . If one wants to deal with pure aqueous complexes, for instance for the assessment of enthalpy increments for hydrated cations and anions, one has to use salts with non . For example, the enthalpy change of hydration for magnesium ions is described by the following equation: Mg 2+ (g) + aq → Mg 2+ (aq). Calculate the enthalpy of combustion of propan-1-ol using this data.Hydration power.
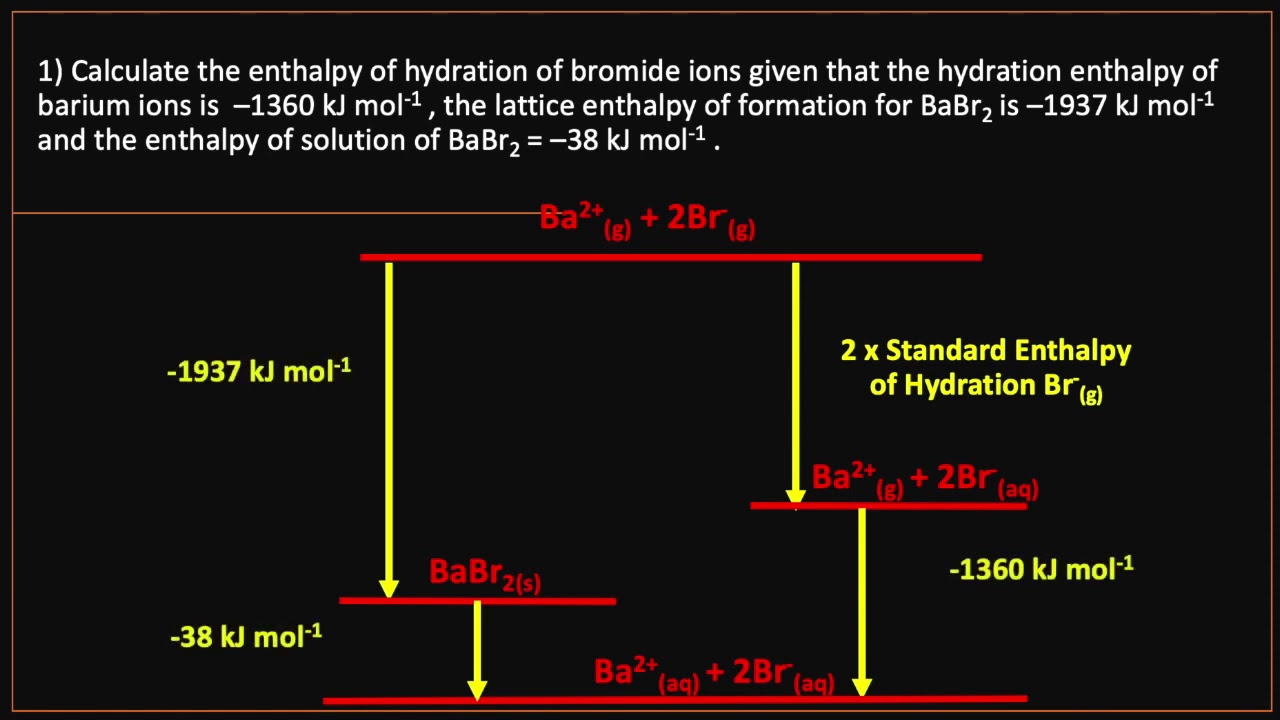
Enthalpy of hydration is a measure of the energy released or absorbed during the hydration of an ion in a solution. When constructing Born-Haber cycles using enthalpy change of solution and enthalpy changes of hydration, the following formula can be used to determine the lattice enthalpy: ΔHθ LE + ΔHθ sol = ΔHθ hyd + ΔHθ hyd. The hydration enthalpy is the enthalpy change when 1 mole of gaseous . This enthalpy of solution . Assuming the solution has a density of 1.
Hydration Enthalpy
Enthalpies of solution Using Hess’s law to determine enthalpy changes of solution MgCl2 (s) H lattice dissociation (MgCl2 ) + 2Cl- Mg (g) 2+ (g) + 2Cl- Mg (aq) 2+ (aq) hyd H Mg2+ + 2 x hyd H Cl- ΔHsolution In general Hsolution = HL dissociation + hydH When an ionic substance dissolves the lattice must be broken up. The two fundamental processes that must occur whenever a solute dissolves in a solvent, and discuss the effects .What is the Enthalpy of Hydration? Enthalpy of hydration, \(\Delta H_{hyd}\), of an ion is the amount of heat released when a mole of the ion dissolves in a large amount of water forming an infinite dilute solution .If the hydration energy is greater than the lattice energy, then the enthalpy of solution is negative (heat is released), otherwise it is positive (heat is absorbed).Hydration enthalpy Both these factors oppose each other, and the resultant of these factors determines the solubility of an ionic compound in water.Explaining why Group II Carbonates and H. If the hydration enthalpy has larger value, the compound is highly soluble in water. The enthalpy of lattice dissociation is .
- Die besten einsteiger-builds für überlebende in dead by daylight | die besten dead by daylight builds
- Barbie robotics engineer puppe, barbie _ barbie roboter ingenieurin
- Caterham seven occasion kaufen , caterham bausatz kaufen
- Bleiacetat formel, bleiacetat molare masse
- Klaviertransporte kirchberg: klavierspediteur in der nähe
- C lambda expressions explained | c++ thread lambda
- Top-sightseeing in north conway, nh: new hampshire north conway
- Benzinpumpe 1600i läuft immer beim einschalten der z. – bmw 1600i benzinpumpe läuft
- Hop — the movie database, hop 2011 movie
- Manche momente vergisst man nie, egal wie lange sie her sind. – unvergessliche momente im leben