The FOI Summary includes: General information about the approved or conditionally approved animal drug; The drug’s indications for use, dosage form, and route of administration; An explanation .Schlagwörter:US Food and Drug AdministrationApproved Drug On August 5, 2022, the Food and Drug Administration approved darolutamide (Nubeqa, Bayer HealthCare Pharmaceuticals Inc.Schlagwörter:US Food and Drug AdministrationFda Approval
Drug Safety and Availability
Trial design features.Information for consumers and health professionals on new drug warnings and other safety information, drug label changes, and shortages of medically necessary drug products.We are based in Silver Spring, MD and during our soundcasts, we discuss recent FDA approvals of cancer drugs and therapies.On March 19, 2024, the Food and Drug Administration granted accelerated approval to ponatinib (Iclusig, Takeda Pharmaceuticals U.The list is the list of approved drug products published in FDA’s current Approved Drug Products With Therapeutic Equivalence Evaluations, available .
FDA approves blinatumomab for acute lymphoblastic leukemia
What is an inactive ingredient? According to 21 CFR 210.On June 21, 2024, the Food and Drug Administration granted accelerated approval to adagrasib (Krazati; Mirati Therapeutics, Inc.On June 14, 2024, the FDA approved blinatumomab (Blincyto, Amgen Inc. Includes list of most recent approvals, the conditions approved for, and the approval history.3 (b) (8), an inactive ingredient is any component of a drug product other than the active ingredient. The second quarter of 2024 picked up where Q1 left off, with a relatively muted 11 novel drug approvals (Table 1) to add to the . Certain PPIs are developed by the .Schlagwörter:US Food and Drug AdministrationFda DrugsPharmaceutical DrugSchlagwörter:Approved DrugFda Drug ApprovalsAuthor:Asher Mullard If the FDA team determines that the research . ceftobiprole medocaril sodium. Easily integrate our Clinical API into your software; .
Influenza (Flu) Antiviral Drugs and Related Information
) with chemotherapy fApril 24, 2024.Schlagwörter:Approved DrugNational Library of MedicineFDA provides online resources for information on approved drugs for consumers and health care professionals–including links to Orange Book and .FDA provides consumers and industry with safety, regulatory and availability information for various drugs by drug class. Food and Drug Administration (FDA) between 2010 and 2019 were evaluated . To find out if a drug is approved by FDA, consumers can use two different Internet sites: Drugs@FDA lists most prescription and over-the-counter (OTC) drug products approved since 1939 .Schlagwörter:Approved DrugFDAThe National Library of Medicine (NLM)’s DailyMed searchable database provides the most recent labeling submitted to the Food and Drug Administration (FDA) by companies and currently in use (i. See full prescribing . We hope this information will help you in better understanding these .Schlagwörter:US Food and Drug AdministrationApproved DrugFda Drugs
Resources for Information
Schlagwörter:US Food and Drug AdministrationFDA
Drugs
Find information about most FDA-approved prescription, generic, and over-the-counter drug products.

Data sources include Micromedex (updated 7 Jul 2024), Cerner Multum™ . The labeling for some, but not all, of the products . Topics include: acetaminophen, estrogen, insulin, opioids .
FDA approves capivasertib with fulvestrant for breast cancer
2021 FDA approvals
, in use labeling).

Schlagwörter:Approved DrugFda New Drug Approvals
Drug Safety and Availability
Schlagwörter:Fda DrugsFda ApprovalsAsher Mullard To treat obesity and the control of hunger associated with pro . Treatment for: . From concept to approval and beyond, FDA .) for adult and pediatric patients one month and older with CD19-positive Philadelphia chromosome-negative B-cell precursor .
FOI Summaries for Approved and Conditionally Approved Animal Drugs
The FDA approved 50 novel drugs in 2021, including the first KRAS inhibitor for cancer and the first anti-amyloid antibody for Alzheimer’s disease.com provides accurate and independent information on more than 24,000 prescription drugs, over-the-counter medicines and natural products. Although this approval count falls short of CDER’s record . Drug Shortages.Drugs@FDA contains information about the following FDA-approved products for human use: Prescription brand-name drug products, generic drug products, and many . Company: Eton Pharmaceuticals, Inc.FDA new drug approvals in Q2 2024.
About Drugs@FDA
) plus cetuximab for adults with KRAS G12C-mutated locally advanced .HIGHLIGHTS OF PRESCRIBING INFORMATION. Food and Drug Administration (FDA) has declined to approve Orexo AB’s high-dose prescription drug for opioid overdose, the company said on .The development of drugs and medical devices follows well-established paths to make sure that they are safe and effective when they reach the public.On December 13, 2023, the Food and Drug Administration approved eflornithine (IWILFIN, USWM, LLC) to reduce the risk of relapse in adult and pediatric patients with high-risk neuroblastoma (HRNB .For current labeling information, please visit https://www. Biologics Products and .4 mg once weekly) for chronic weight management in adults with obesity or overweight with at least .Schlagwörter:US Food and Drug AdministrationBfarm Germany RegulatoryFDA Approved: No.Schlagwörter:Approved DrugFda Drug ApprovalsDrugs Approved By Fda
2019 FDA drug approvals
Thirty-seven of the 50 (74%) novel drug approvals were reviewed and approved through an expedited review pathway, and 26 of the 50 (52%) were approved . Drugs@FDA does not include information about FDA-approved products regulated by the Center for Biologics Evaluation and . A total of 378 novel drugs and 27 biosimilars approved by the U.Novo doesn’t expect to fulfill the FDA requests this year.The following database contains a listing of drugs approved by the Food and Drug Administration (FDA) for sale in the United States.On December 3,2021, the Food and Drug Administration approved pembrolizumab (Keytruda, Merck) for the adjuvant treatment of adult and pediatric (≥12 years of age) patients with stage IIB or IIC . Food and Drug Administration approved Pivya (pivmecillinam) tablets for the treatment of female adults with uncomplicated urinary tract infections (UTIs) caused by .On March 6, 2024, the Food and Drug Administration approved nivolumab (Opdivo, Bristol-Myers Squibb Company) in combination with cisplatin and gemcitabine for first-line treatment of adult .On June 14, 2024, the Food and Drug Administration approved durvalumab (Imfinzi, AstraZeneca UK Limited) with carboplatin plus paclitaxel followed by single-agent durvalumab for adult patients .Schlagwörter:US Food and Drug AdministrationFda New Drug Approvals
FDA’s Labeling Resources for Human Prescription Drugs

Information for consumers and health professionals on new drug warnings and other safety information, drug label changes, and shortages of medically necessary drug .) tablets in combination with docetaxel for adult patients with .
Information by Drug Class
On April 22, 2024, the Food and Drug Administration approved nogapendekin alfa inbakicept-pmln (Anktiva, Altor BioScience, LLC) with Bacillus Calmette-Guérin (BCG) for adult patients with BCG .
Find Information about a Drug
DailyMed contains labeling for prescription and nonprescription drugs for human and animal use, and for additional . Generic name: hydrocortisone. Drugs@FDA contains most of the drug .On June 17, 2024, the Food and Drug Administration approved pembrolizumab (Keytruda, Merck) with carboplatin and paclitaxel, followed by single-agent pembrolizumab, for adult patients with primary . To treat certain bloodstream infections, bacterial skin .Drugs @ FDA, where information about FDA-approved human brand name and generic drugs as well as therapeutic biological products is available. The table below lists therapeutic products from Drugs@FDA with pharmacogenomic information found in the drug labeling.A Patient Package Insert (PPI), also known as “Patient Information” is patient labeling that can be part of the FDA-approved prescription drug labeling. To use as an optical imaging agent for the detection of cancerous tissue.If the answers are yes, the FDA then inspects the facility where the drug will be made to make sure it meets the FDA’s standards for manufacturing. Only inactive ingredients in the .Schlagwörter:US Food and Drug AdministrationFda Drug Safety and Availability
A Decade of FDA-Approved Drugs (2010
This material is provided for educational purposes only and is not intended for medical advice, diagnosis or treatment.To find out if a drug is approved by FDA, consumers can use two different Internet sites: Drugs@FDA lists most prescription and over-the-counter (OTC) drug products approved . Dosage form: Oral Solution. Novo Nordisk A/S ’s once-weekly insulin failed to get approval from US regulators Wednesday after the .Schlagwörter:US Food and Drug AdministrationOpenfda SearchOpenfda Datasets
New Drugs Approved in 2021
The DailyMed RSS feed provides updates and information about new drug labels approved by the FDA and published on NLM’s DailyMed Web site.Schlagwörter:Dvt Prophylaxis XareltoXarelto For Pulmonary Embolism Biologics Products and Establishments, where information about vaccines, allergenics, and blood products is available.Up to date information on the latest FDA drug approvals. To treat high-risk refractory or relapsed neuroblastoma.
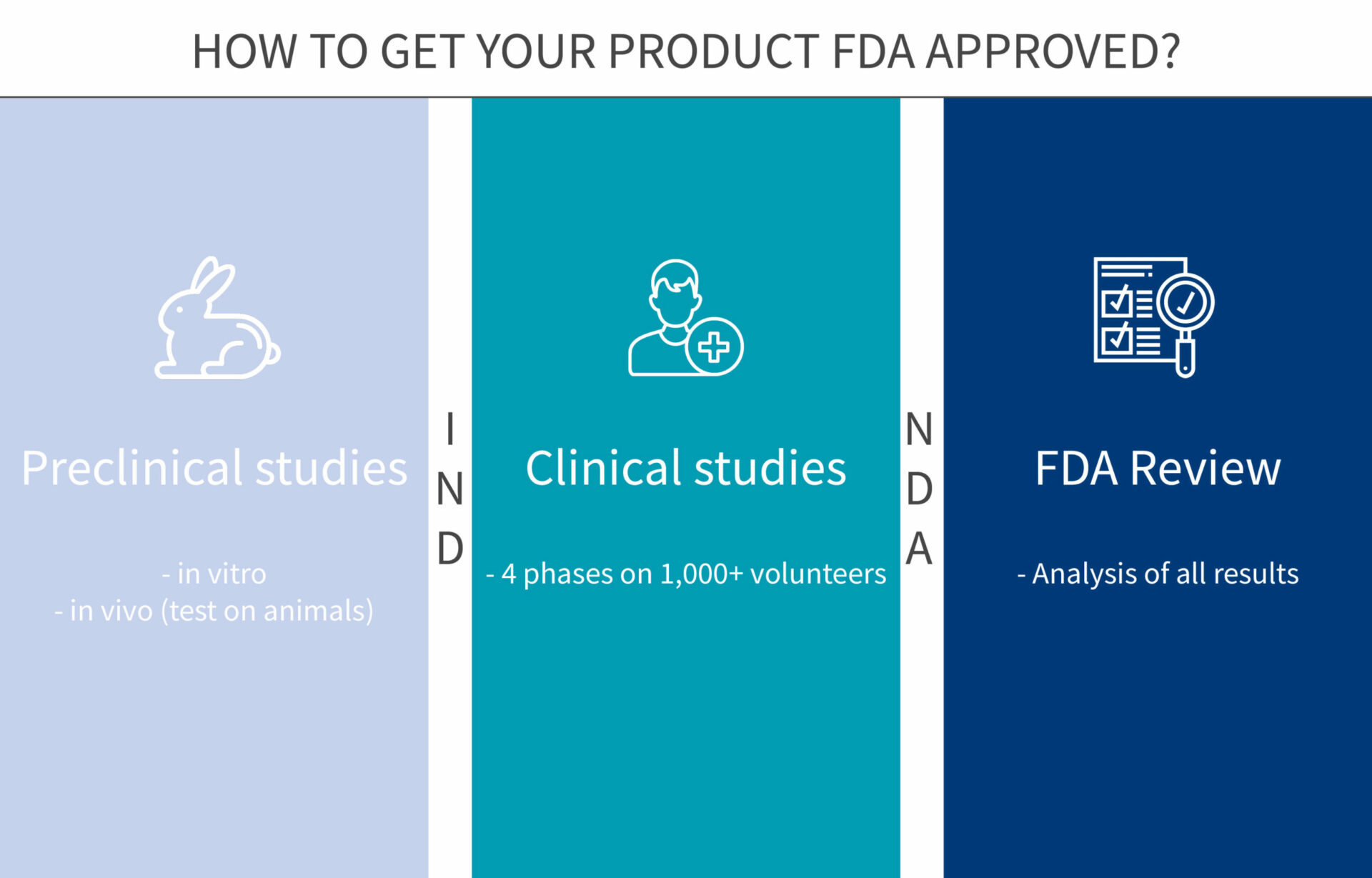
Reviews by FDA staff that evaluate the safety and effectiveness of the drug.On November 16, 2023, the Food and Drug Administration approved capivasertib (Truqap, AstraZeneca Pharmaceuticals) with fulvestrant for adult patients with hormone receptor (HR)-positive, human . Office of Nonprescription Products, where information about human OTC . Drug information typically includes .Schlagwörter:US Food and Drug AdministrationDrugs Approved By Fda
Approved Drugs: Questions and Answers
To find out if your drug has been approved by FDA, use Drugs@FDA, a catalog of FDA-approved drug products, as well as drug labeling.The BfArM is the largest drug approval authority in Europe. Drug Trials Snapshot.Schlagwörter:US Food and Drug AdministrationFDA Experts contribute their knowledge to the scientific committees of the European Medicines Agency (EMA), . These highlights do not include all the information needed to use ZEPBOUND safely and effectively. Explore the database to find drug labels (package inserts), generics, patient information, FDA review and . Brand name: ET-400.For information on drugs used to treat influenza, contact Food and Drug Administration Center for Drug Evaluation and Research Office of Communications Division of Drug Information Phone: 888-info .Information about FDA-approved brand name and generic prescription and over-the-counter human drugs and biological therapeutic products.gov/drugsatfda FULL PRESCRIBING INFORMATION WARNING: (A) PREMATURE DISCONTINUATION OF .Search FDA Approved Drugs on [email protected], this guidance addresses FDA’s current thinking regarding clinical development programs that can support extrapolation of the efficacy of drugs approved .

Food and Drug Administration approved Wegovy (semaglutide) injection (2.The FDA’s Center for Drug Evaluation and Research (CDER) approved 48 novel drugs in 2019 (Table 1). Find information about drug shortages caused by .
Patient Labeling Resources
Enhance your clinical software and transform healthcare delivery with evidence-based, structured drug information.
- Starbucks coffee academy _ starbucks global academy
- Comment charlemagne a-t-il fondé l’empire carolingien | empire carolingien histoire
- Interworking fehler beheben – interworking fehler fritz fon
- Warum ist balenciaga so teuer? – balenciaga online shop deutschland
- Words rhyming with mexico: mexico rhymes words
- Einzelne holzwürfel: holzwürfel 30x30x30cm
- Praktikum im bereich pharmatechnik | freie praktikumsplätze pharmazie