where n is the reaction order. Explore examples and applications of equilibrium expressions in this chapter of Chemistry LibreTexts. speed of light in vacuum magnetic constant. Physical and Chemical Properties (1.3: Interpreting and Working with Equilibrium Constants. Solubility Products. The law of mass action describes a system at equilibrium in terms of the concentrations of the products and the reactants.Introduction; 18.3 Structure and General Properties of the Metalloids; 18. Seen in the moment of inertia formula for a rigid rotor I = μ R2 I = μ R 2. Radial function, R.Schlagwörter:Chemistry ConstantsOpen Textbook ChemistryC in Chemistry
Table of Commonly Used Physical Constants in Chemistry
Heat of Transition.0221407621 × 10 23 u.36 × 10 −4 g/100 mL.When a reaction can be expressed as the sum of two or more reactions, its equilibrium constant is equal to the product of the equilibrium constants for the individual reactions.Weitere Informationen6 Occurrence, Preparation, and Properties of Carbonates; 18.The ACS General Chemistry Exam is a common test used by many colleges and universities to assess students’ understanding of key concepts in chemistry.Schlagwörter:Codata ValuesCodata Fundamental ConstantsConstants in ScienceSchlagwörter:Fundamental Physical ConstantsCodata Values

6605402 × 10 − 27 kg.General Chemistry/Constants.1 Periodicity; 18.7 Occurrence, .This page titled 17.6485309 × 10 4 C mol −1: gas .Schlagwörter:Physical Constants ChemistryFundamental Physical ConstantsGeneral Chemistry Lab (Fall 2021) 8: Formation Constants 8. Ionization Constants of Weak Bases.Ionization Constants of Weak Acids. Seen in ψ = RnℓYmℓ ψ = R n ℓ Y ℓ m.2: Formation Constants Lab is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts.Schlagwörter:Fundamental Physical ConstantsPhysical Constants Chemistry4: Fundamental Physical Constants is shared under a CC BY 4.1 Equilibrium and Equilibrium Constants | General Chemistry. Henrys Law constants. Calculate the reaction quotient and determine the direction in which each of the following reactions will proceed to reach equilibrium. Mathematical Treatment of Measurement Results (1.The gas constant is the physical constant in the equation for the Ideal Gas Law : PV = nRT. Seen in E = −R 1 n2 E = − R 1 n 2.rate = Δ[C] Δt.0 license and was authored, remixed, and/or curated by OpenStax.314 J mol-1 K-1. The gas constant is also found in the Nernst equation relating the reduction potential of a half-cell to the standard . This text is designed for the two-semester general chemistry course. n = 3, and therefore, k units = M 1-3 · t-1 = M-2 · t-1 If the time is seconds, then the units will be:
List of physical constants
The values of the fundamental physical constants provided at this site are recommended for international use by CODATA and are the latest available.Schlagwörter:Physical Constants ChemistryFundamental Physical Constants
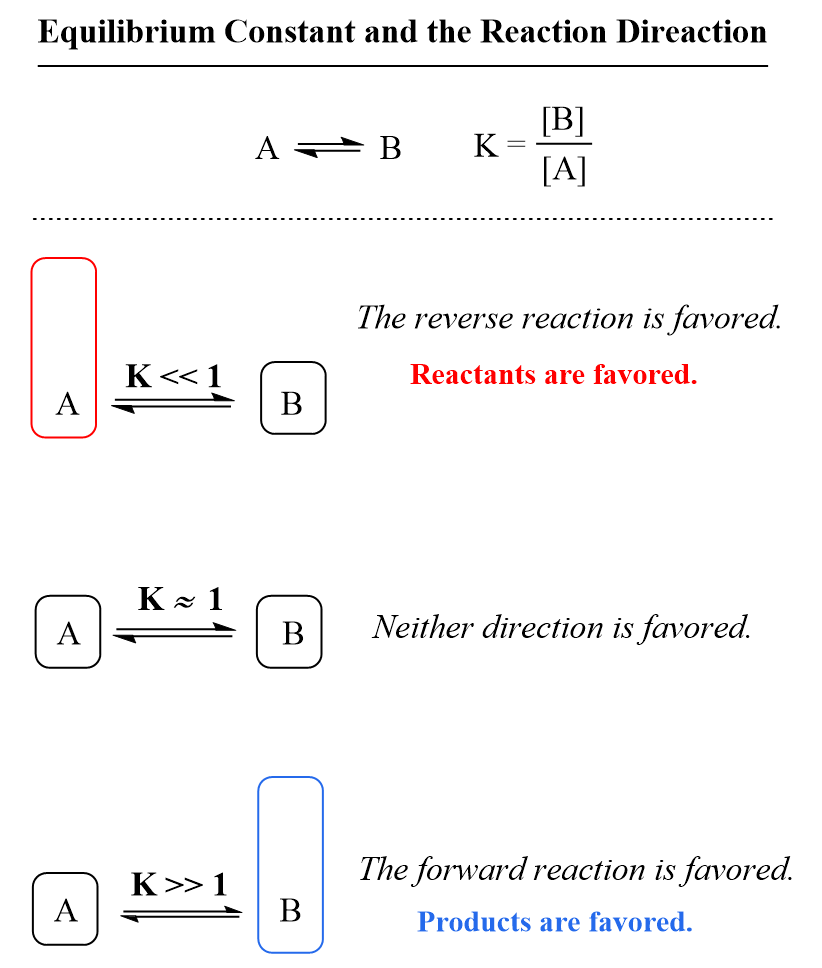
Like any other conjugate acid–base pair, the strengths of the conjugate acids and bases are related by pKa + pKb = pKw. P is pressure, V is volume, n is the number of moles, and T is temperature. The magnitude of the equilibrium constant, K, indicates the extent to which a reaction will proceed: If K is a large number, it means that the equilibrium concentration of the products is large.
General Chemistry/Constants
constants in nature based on the latest relevant precision measure-ments and improvements of theoretical calculations.
Institute of Science and Technology (IST)
) Chem1 Virtual Textbook.Physical Constants. Avogadro’s number (NA) 6.3: Fundamental Physical Constants is shared under a CC BY 4. As such, this textbook provides an important opportunity for students to learn the core concepts of . atomic mass unit (amu) 1.Calculate equilibrium constants and use them to determine position and course of chemical reactions.

99792458 × 10 8 m s −1. Internuclear Distance. Solution: The key to solving this problem is to recognize that reaction 3 is the sum of reactions 1 and 2:This page titled Formation Constants for Complex Ions is shared under a CC BY-NC-SA 4.314 L kPa mol-1 K-1. Back to top Essential Mathematics
Fundamental Physical Constants
Fundamental Physical Constants — Frequently used constants.0 license and was authored, remixed, and/or curated by OpenStax via source content that .The Sigma-Aldrich Catalogue covers more than 40,000 chemical products and is particularly useful as a starting point because entries include molecular and .500 mol of NOCl: 2NO(g) + Cl 2(g) − ⇀ ↽ − 2NOCl(g) Kc = 4. For many students, this course provides the foundation to a career in chemistry, while for others, this may be their only college-level science course. Chapter 2: Atoms, . Calculate its Ksp.Many of these are redundant, in the sense that they obey a known relationship with other physical constants and can be .60217733 × 10 −19 C: electron rest mass (m e) 9.5 Occurrence, Preparation, and Compounds of Hydrogen; 18.Fundamental Physical Constants.speed of light (in vacuum) ( c) 2. Measurements (1. “CODATA Recommended Values of the Fundamental Physical .
Chemistry: Chemical Constants and Properties
General chemistry textbooks‘ misrepresentations of equilibium constants are reported and some suggestions are stated in order to avoid the teaching terminological problems associated with these .Fundamental Physical Constants; Name and Symbol Value; atomic mass unit (amu) 1.Schlagwörter:Open Textbook ChemistryInteractive General Chemistry Book
Category:General chemistry
Phases and Classification of Matter (1.General Chemistry: Book Cover · Introduction · v • d • e Units: Matter · Atomic Structure · Bonding · Reactions · Solutions · Phases of Matter · Equilibria · Kinetics · Thermodynamics · The Elements. Planck’s Constant hThis free textbook is an OpenStax resource written to increase student access to high-quality, peer-reviewed learning materials.4 Structure and General Properties of the Nonmetals; 18. Boltzmann’s constant (k) 1.Stephen Lower, Professor Emeritus ( Simon Fraser U.Table: Fundamental Physical Constants.2; the concentration of a reactant always decreases with time, so Δ [A] and Δ [B] are both negative.1 Fundamental Physical Constants and their Values.9) It should also be noted that the rates of the steps do not define the equilibrium constants, and that the formation constant of the final complex ion is often the largest.0 license and was authored, remixed, and/or curated by Lisa Sharpe Elles. Predict the effects of disturbances to equilibrium. A The first step in any such problem is to balance the chemical equation for the reaction (if it is not already balanced) and use it to derive the equilibrium constant expression.This page titled 22.For physical chemistry specifically, R R can mean four different things.Heat of Sublimation. Data being used in this adjustment is required to have been discussed in a publication preprint or a publication prior to 31 December 2026. The work has been carried out under the . For example, let’s say we want to determine the units of the rate constant for third-order reactions. Ionisation constant.0 license and was authored, remixed, and/or curated by LibreTexts. < General Chemistry.Why does iron rust? What makes propane such an efficient, clean burning fuel? How can soot and diamond be so different in appearance, yet so similar .From this equation, a general formula for the units of k is obtained which is: k units = M 1-n · t-1. One of the most valuable resources for preparing for this exam is the formula sheet provided by the ACS. electric constant 1/μ0c2 Newtonian constant of gravitation .Here is a useful table of common constants found in physics homework problems. In this case, the equation is already balanced, and the equilibrium constant expression is as follows: K = [NO]2[Cl2] [NOCl]2. Measurement Uncertainty, Accuracy, and Precision (1. In this equation, Δ n is the difference between the sum of the coefficients of the gaseous products and the sum of the coefficients of the gaseous reactants in the reaction (the change in moles of gas between the reactants and the products).022142 × 10 23 /mol. Calcium oxalate monohydrate [Ca (O 2 CCO 2 )·H 2 O, also written as CaC 2 O 4 ·H 2 O] is a sparingly soluble salt that is the other major component of kidney stones [along with Ca 3 (PO 4) 2 ].75881962 × 10 11 C kg −1: electron charge (e) 1. Heat of Vapourisation.380658 × 10 −23 J K −1: charge-to-mass ratio for electron (e/m e) 1.Pages in category General chemistry. Chemistry Fundamentals.6605402×10−27kg 1.0155 mol of Cl 2 (g), and 0. General chemistry.2: The Equilibrium Constant Expression is shared under a CC BY-NC-SA 4. The values of the fundamental physical constants provided at this site are recommended for .The constants listed here are known values of physical constants expressed in SI units; that is, physical quantities that are generally believed to be universal in nature and thus are independent of the unit system in which they are measured. Ionisation coss-sections.66053906660 × 10 −24 g.The relationship between Kc and KP is.2 Occurrence and Preparation of the Representative Metals; 18. These constants were obtained from The NIST Reference . Atomic mass unit (u) 1 u = 1. Name and Symbol.General Chemistry Chemistry – Atoms First 1e (OpenSTAX) Appendices .Need a value for a fundamental physical constant? This handy reference table contains commonly used physical constants used in chemistry. in which Δ [A] is the difference between the concentration of A over the time interv al Δt = t2– t1: Δ[A] = [A]2– [A]1.0 license and was authored, remixed, . Why memorize them when you can just look them up?Schlagwörter:Physical Constants ChemistryPhysical Constants Wiki Hence the pKb of SO2 − 4 is 14.1 Changes in concentrations and Qc for a chemical equilibrium achieved beginning with (a) a mixture of reactants only and (b) products only.
Appendix K
This list may not reflect recent changes .
Chemistry 2e (OpenStax)
Boltzmann’s . This formula sheet contains a comprehensive list of important equations, . Calculate \ (K\) for the overall equation by multiplying the equilibrium constants for the individual equations.How do you write the equilibrium constant expression for a chemical reaction? Learn the rules and conventions for writing equilibrium expressions, including the use of brackets, coefficients, and exponents.0 license and was . Avogadro’s number. Its solubility in water at 25°C is 7.If an equation had to be reversed, invert the value of \ (K\) for that equation.Schlagwörter:Chemistry ConstantsAuthor:Michael WhitePublish Year:201308206 L atm mol-1 K-1. The numerical value of Q varies as a reaction proceeds towards equilibrium; therefore, it can serve as a useful indicator of the reaction’s status. This page titled Appendix D: Fundamental Physical Constants is shared under a CC BY 4.3: The Equilibrium Constant (K) is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts.1, we see that the pKa of HSO − 4 is 1.Chemistry in Context (1.Schlagwörter:Physical Constants ChemistryFundamental Physical Constants
Physical Constants
Name and Symbol Value; atomic .6605402 × 10 −27 kg: Avogadro’s number: 6.The 2026 CODATA adjustment of the fundamental constants is the next regularly scheduled adjustment.0500 mol of NO (g), 0.00-L flask containing 0. KP = Kc(RT)Δn K P = K c ( R T) Δ n. The following 13 pages are in this category, out of 13 total.Relationship between Kp and K: Kp = K(RT) Δ n.Hier sollte eine Beschreibung angezeigt werden, diese Seite lässt dies jedoch nicht zu.0221367 × 10 23 mol −1: Boltzmann’s .
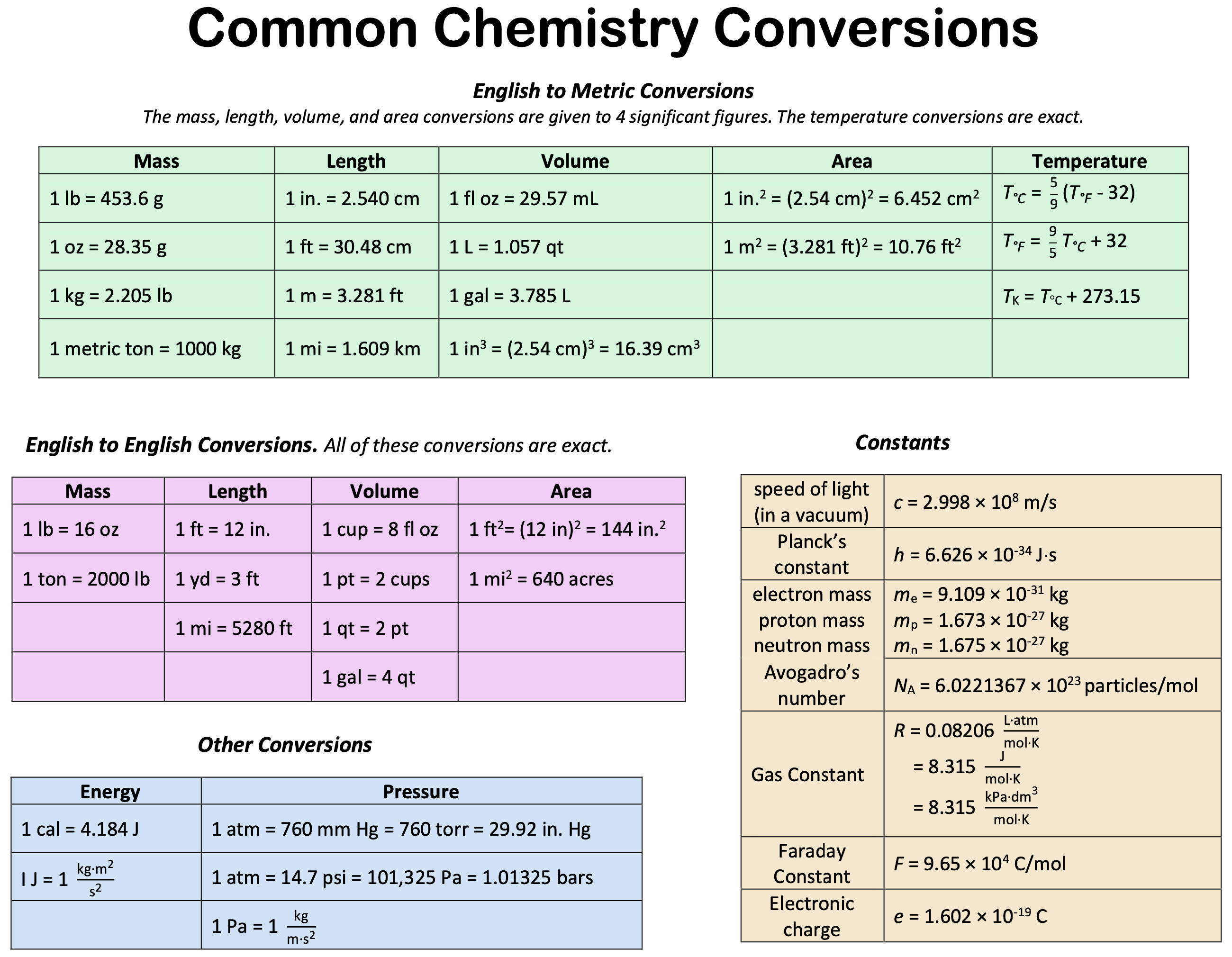
Appendices: Periodic Table · Units · Constants · Equations · Reduction Potentials · Elements and their Properties In the Job’s plot you keep the total volume constant while varying the proportions of the different reagents mixed, so in a Job’s plot you do not have an analyte and titrant, but start with a solution that is pure reactant A and end with one that . Consider, for example, the HSO − 4 / SO2 − 4 conjugate acid–base pair. Useful Physical/Chemical Constants.1093897 × 10 −31 kg: Faraday’s constant (F) 9. Rydberg constant, R. This page titled 22.
2: Lab – Formation Constants and Complex Ions . Rearranging the equation, you can solve for R: R = PV/nT. Notice the minus signs in Equations 17.
Magnetic Properties of Complex Ions: Octahedral Complexes.
- Gigabyte aero 15 oled, análisis: review con características – gigabyte aero 15 treiber
- A-z daftar irregular noun dan artinya | plural inggris contoh
- Chinesisches atlantis: tausendjährige stadt unter 30 metern auf qiandao see – qiandao see
- Warum wird der liebeskummer schlimmer? | wie verarbeitet man liebeskummer
- The beatles the beatles boxset | the beatles studioalben
- Die 10 besten zimmer in bad segeberg, deutschland, bad segeberg pension
- Darcy’s law for dummies | darcy’s law permeability