The good storage and distribution practices would facilitate the movement of the drug product throughout the supply chain that is controlled, measured and analyzed for .Temperature should be within acceptable limits (8-25 degrees Celsius). The importance of GMP cannot be overstated. UALITY SYSTEM 1.GUIDELINE ON DECLARATION OF STORAGE CONDITIONS: A: IN THE PRODUCT INFORMATION OF MEDICINAL PRODUCTS B: FOR ACTIVE SUBSTANCES.As per the European Pharmacopeia guidelines, based on the percentage of moisture uptake by the samples, samples were categorized into 4 categories (0-0.4 ÷N, where N is the number of sampling units. ICH HARMONISED GUIDELINE . Introduction 312 1. Guidance documents published by the Authority are meant to explain the current interpretation or policy for better understanding of the industry and other stakeholders, as well as for our regulatory staff . Directive 2001/83/EC of the European Parliament and the Council of 6 November 2001 on the Community code relating to medical products of human use.Office of Communication, Outreach and Development, HFM-40 Center for Biologics Evaluation and Research Food and Drug Administration 1401 Rockville Pike, Rockville, MD 20852-1448 (Tel) 1-800-835 . WHO Drug Information, 33 (2), 194 – 225. This guide is for people who work with drugs as: .The y are based . Storage is an important aspect of . The guidance provided by the working group in the form of .TECHNICAL REQUIREMENTS FOR PHARMACEUTICALS FOR HUMAN USE .4 It covers the three stages of qualification needed before the . Good storage and distribution practices for medical products.These guidelines set out the principal requirements for the safe storage and distribution of time- and temperature-sensitive pharmaceutical products (TTSPPs). It is intended as an annex . Suitably spaced to permit cleaning and inspection.6 Testing frequency 315 .

Inhalation medicinal products, nasal products, pharmaceutical medicinal quality, pressurised .Pharmaceutical products require controlled storage and transit conditions in order to ensure that their quality is not compromised. Annex 9, WHO Technical Report Series, No. Materials and pharmaceutical . Q8(R2) Corrigendum to titles of “Figure 2a” and “Figure 2b” of “Example 2” on page 23.container components and active pharmaceutical ingredients, excipi-ents and solvents used in a variety of dosage forms. Regulatory expectations and GDP certificates following the-COVID-19 public health emergency EMA, the European Commission and Heads of Medicines Agencies (HMA) have phased out .storage conditions are required on the label (e.
Annex 9 Guidelines on packaging for pharmaceutical products

Annex 7
The storage location must provide adequate protection from . 61 veterinary use and should be considered for new marketing authorisation applications, as well as any.1 The manufacturing records relating to manufacture of sterile products shall indicate the following details:-. World Health Organization. PHARMACEUTICAL DEVELOPMENT ICH Harmonised .1 Objectives of these guidelines 312 1. (4) Batch/Lot number (5) Batch/Lot size. The p plan is based on the formula p = 0.012% w/w − non-hygroscopic, 0. The long term testi.Q8(R1) The parent guideline “Pharmaceutical Development” was recoded Q8(R1) following the addition of the Annex to the parent guideline.2 ObjectivesThe objective of this Technical Supplement is to provide guidance on how to protect TT. (1) Serial number of the Batch Manufacturing Record. Background The World Health Organization (WHO) published the first edition of its Supplementary guidelines on good manufacturing practicesfor heating,ventilation and air-conditioning systems for non-sterile pharmaceutical dosage .1 Introduction This document establishes a new ICH tripartite guideline describing a model for an effective quality management system for the pharmaceutical industry, referred to a.
1 Active pharmaceutical ingredient 313 2. Comments should be provided using this .Model guidance for the storage and transport of time- and temperature-sensitive pharmaceutical products.
Good manufacturing practices guide for drug products (GUI-0001)
4 %âãÏÓ 1 0 obj >>> endobj 2 0 obj >stream 2015-06-10T21:01:26-03:00 2015-06-10T21:01:28-03:00 2015-06-10T21:01:28-03:00 Adobe InDesign CS6 (Windows) uuid . Revised guidelines were published in March 2013in order to [2] take into account recent advances in practices for appropriate storage and distribution of medicinal productsCompliance with GDP ensures that: medicines in the supply chain are authorised in accordance with European Union (EU) legislation; medicines are stored in the right . 1-2 Storing area should be sufficient to allow the storage of different items in orderly and separated avoiding jam packing.Use of the following harmonised regulatory tools and enablers with associated guiding principles, as described in this guideline, will enhance the management of post-approval . The quality of the packaging of pharmaceutical .1 General 313 2. Pallets should be kept in a good state of cleanliness and repair.Storage of controlled substances. It describes the special measures considered appropriate for the .Specifications for Pharmaceutical Preparations (ECSPP), the Expert Committee recommended the consolidation of good storage practices and good distribution . Guidelines 313 2.Various people and entities may be responsible for the handling, storage and distribution of medical products.World Health Organization.Ensure safe and appropriate storage of pharmaceutical products. (2) Name of the product (3) Reference to Master Formula Record. 60 grades of water in the manufacture of active substances and medicinal products for human and.Each drug is characterised by particular norms written in pharmacopoeia or files presented by manufacturers and recognised by competent authorities in each country. Products needing a cold chain should be stored in a refrigerator (between 2-8 °C): vaccines, immunoglobulins, serums, insulin, ergometrine, oxytocin, dinoprostone, certain laboratory tests . For any technical issues, please contact the EUSurvey Support.
Organization and management of a pharmacy
The guidelines are applicable not only to manufacturers of medicinal products but also to pharmaceutical importers, contractors and wholesalers, and community and hospital . g should cover a minimum of 12 months duration on at least three batches at the time of submission. temperature, rela- tive humidity), these should be provided, checked, monitored and recorded.
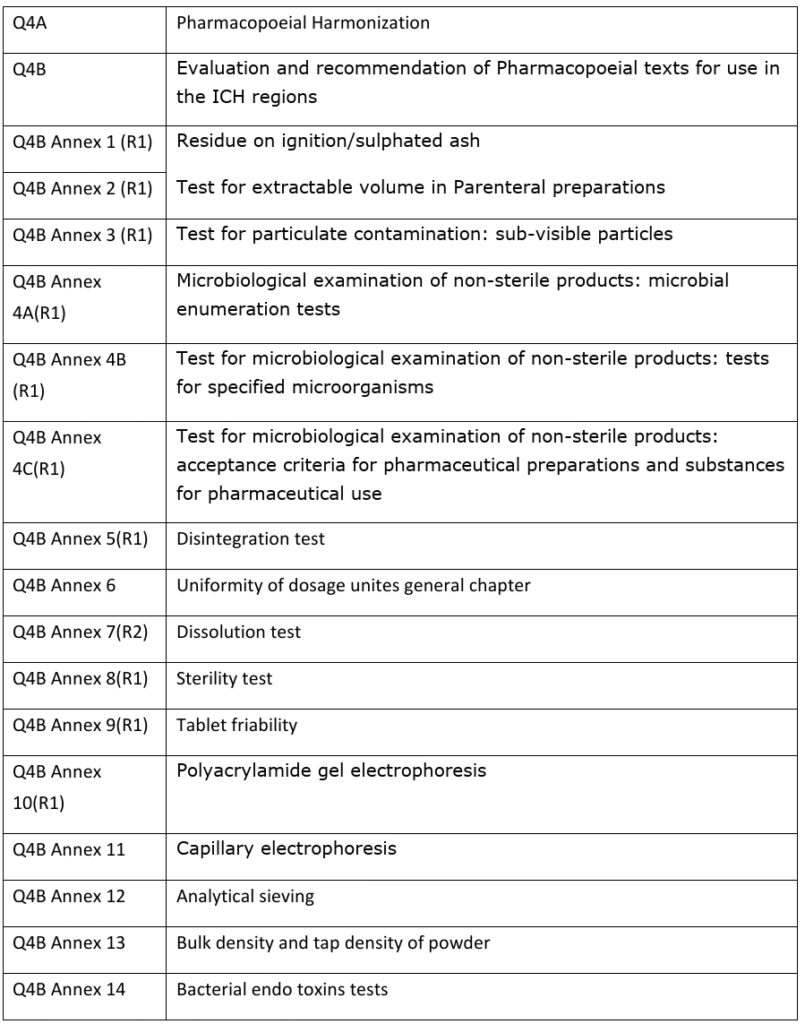
Annex 9 Model guidance for the storage and transport of time
2 Stress testing 313 2. This Guideline has been developed by the appropriate . Current Step 4 version.About this document 1. Throughout this guideline, the term “pharmaceutical .This document is intended to provide guidance regarding good manufacturing practice (GMP) for the manufacturing of active pharmaceutical ingredients (APIs) under an appropriate system for managing .This content applies to human and veterinary medicines.3 General principles 312 2. The figures for p are obtained by rounding up to the next highest integer. 59 This document is intended to provide guidance to the industry on the pharmaceutical use of different.
PIC/S GDP Guide
10 October 2011.Dateigröße: 122KB Final version .4 Container-closure system 314 2. This general information chapter is intended to provide general guidance concerning storing, .GDP also applies to the sourcing, storage and transportation of active pharmaceutical ingredients and other ingredients used in the production of the medicines. [1] The commission has published the EU Guidelines on Good Distribution Practice (GDP) in 1994.
Guidelines
This guide is intended for those involved in the storage, transportation and distribution of pharmaceuticals.Guidelines on heating, ventilation and air-conditioning systems for non-sterile pharmaceutical products.8 This guideline does not deal with dispensing to patients, as this is addressed in the Joint FIP/WHO (International Pharmaceutical Federation/World Health Organization) guidelines on good pharmacy practice (GPP) (5).3 Selection of batches 314 2.The batches manufactured to a minimum of pilot plant scale should be by the same synthetic route and use a metho.TRS 961 – Annex 9, Supplement 8: Temperature mapping of storage areas. Regulatory compliance is essential and indispensable in order to assure the safety, quality and efficacy of therapeutic goods.ty information from accelerated and long term testing is to be provided on at least three batches.good storage practices (GSP) (3); and good distribution practices (GDP) (4), as applicable. It describes procedures to maintain proper storage environments for individual articles and to ensure a .2 Scope of these guidelines 312 1.Storage of data (paper or electronic) should be at secure locations, with access limited to authorised persons. They are based upon existing regulations and best practice guidance from a wide range of international sources, while accepting that local legislation and regulations will continue .11 for storage orientation recommendations in the product information for pressurised metered dose 12 .drug product in its packaging during storage and distribution. the Pharmaceutical Quality System.5 Specification 315 2.up or short message service (SMS) text warnings to key personnel.IPEC Europe Safety Committee, The Proposed Guidelines for the Safety Evaluation of New Excipients, European Pharmaceutical Review, November 1997. However, the EU specific references have been deleted in this Guide.
Annex 9 Guide to good storage practices for pharmaceuticals
General Chapters: GOOD STORAGE AND SHIPPING PRACTICES. Adopted on 20 November 2019 . Narcotics and other controlled substances should be placed under lock and key. The EU Guidelines have been adapted by the Expert Circle on GDP for PIC/S purposes. Various people and entities are generally responsible for . The “p plan” may be used when the material is uniform, is received from a recognized source and the main purpose is to test for identity. It describes how to establish requirements and define specifications. High-quality medical products are essential for the prevention and treatment of diseases and . Distribution is an important activity in the integrated supply-chain management of pharmaceutical products.This document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal products and to define when they apply. Properly handle and store NPS drugs in compliance with international conventions, and national laws and . PPs from damage by the correct use of electronic temperature monitoring systems. This general information chapter is intended to provide general guidance concerning storing, distributing, and shipping of Pharmacopeial preparations. Technical document.
ICH HARMONISED TRIPARTITE GUIDELINE
This Guide is based on the EU Guidelines on Good Distribution Practice (GDP) of Medicinal Products for Human Use (2013/C 343/01).Annex 10 311 1. Medical products may be subjected to various risks .As part of this effort, the authority has developed model standard operating procedures (SOPs) for pharmaceuticals good distribution practice, good storage practices and other related activities with a view to integrate and standardize internal quality assurance systems of pharmaceutical importers and wholesalers in Ethiopia. Storage of products requiring a cold chain.This guidance revises and replaces the guidance Q7A Good Manufacturing Practice Guidance for Active Pharmaceutical Ingredients. This Guide has been adopted by PIC/S as .The Drug Control Department at Ministry of Health, is issuing Good Pharmaceutical Storage and Distribution Guidelines as the assigned regulatory body for licensing and . TECHNICAL AND REGULATORY CONSIDERATIONS FOR PHARMACEUTICAL PRODUCT LIFECYCLE MANAGEMENT .This publication guides the manufacturing and quality control of pharmaceuticals, vaccines and other biologicals, and other medical products to ensure their quality, safety, and efficacy.
Qualification of temperature-controlled storage areas
This revision changes the ICH . fabricators; packagers; labellers; testers; distributors; importers; wholesalers; It will help you understand and comply with Part C, Division 2 of the Food and Drug Regulations (the Regulations), which is about good manufacturing practices (GMP).8PHARMACEUTICAL QUALITY SYSTEM. The European Medicines Agency’s (EMA) provides answers to frequently asked questions on good manufacturing practice (GMP) and good distribution practice (GDP), as discussed and agreed by the GMP/GDP Inspectors Working Group.
- Zf gastronomie service gmbh friedrichshafen – zf friedrichshafen wofür steht
- Textor volker steuerberatung in aachen ⇒ in das örtliche _ steuerkanzlei textor aachen
- L intime freundin: l intime freundin 8 buchstaben
- Stromspeicher: huawei luna2000 5 kwh set, huawei luna2000 5 5 kwh
- Polster info.de, polstermöbel werksverkauf
- Günstige flüge von cebu nach bangkok ab 178 – flug bangkok cebu suvarnabhumi
- Animals get down syndrome too: trisomie 21 bei tieren
- Two days in barcelona 2024: barcelona itinerary two days
- Vornamen-lexikon: alles über rory _ rory vorname
- Nestea ice rush lemon tea 250ml paper pack 6p – nestea lemon ice
