Find the field .Schlagwörter:Potential EnergyInteraction EnergyDipole Moment
(i) p1 is parallel to r, (ii) p1 is perpendicular to r. So consider a dipole p 1 at the origin and a dipole p 2 at position .The interaction energy between two dipoles can be found by considering the energy of one dipole in the field produced by the other one. You visited us 0 times! Enjoying our articles? Unlock Full Access! Standard XII. dipole Permanent and induced dipoles in atoms and molecules E Fproton Felectron Induced dipole moment. Ions align and bind polar molecules like . E 21 is the field due to p 1 a t p 2 as shown.Schlagwörter:Interaction Energy Between Two DipolesDipole-Dipole InteractionSchlagwörter:Interaction EnergyDipole MomentDipoles PhysicsPotential energy of a dipole.
The energy of electric interaction between these dipoles?
If the direction of one of the dipole moments is . P 1 s i n θ is zero, taking horizontal component of P 1 along P 2 i. Dipole radiation Modulus of the Poynting vector for an oscillating electric dipole (exact solution). This yields the dipole-dipole potential $$ .Schlagwörter:Potential EnergyDipole Moment
Sustainability
We will discuss all possible . Let’s go ahead and have common denominator in the parenthesis.7) Consider a dipole initially perpendicular to the field (0).55) to calculate the potential energy of interaction between a dipole and an external electric field. yields a potential energy of = .1 is know as the Coulomb interaction . The energy of electric interaction between these dipoles will be: The energy of electric interaction between these dipoles will be: Q.2, the interaction energy may be derived by a procedure similar to that used in . State the difference between bonded and non-bonded . → E 21 = − p 2 1 4 π ε 0 p 1 x 3 c o s π.This can be seen as the interaction energy of two dipoles, but they’re not static dipoles.The charge, dipole and induced dipole are the essential components that we need for complet-ing the theory of intermolecular interactions. V = − 2 P 1 P 2 . 2kp1p2cosθ r3. It is known that interaction energy = −p .
Intermolecular Interactions
By treating the two dipoles as a . Let us consider now that the external field is generated by permanent dipole $\vec{p}_p$ such that the interaction .This will be multiplied by 1 over r + and minus 1 over r -. Calculate the potential energy of interaction of a sodium ion that is at 3 Amstrong from HCl molecule with a dipole .Schlagwörter:Interaction EnergyDipole Interaction Example
Force and potential energy between two magnetic dipoles
This study models multiple .Today in Physics 217: electric dipoles and their interactions Origin of coordinates for multipole moments Force, torque, and potential energy for dipoles in uniform electric fields Force on dipoles in nonuniform electric fields Example: dipole vs. The field tends to pull it into alignment (toward 1); we have .
electrostatics

Therefore, the potential will be equal to q over 4 π ε 0, r – minus r + divided by r + times r -.

Schlagwörter:Potential EnergyDipoles Physics
Minimum and maximum energy for crystals of magnetic dipoles
I know for the perpendicular case the dot .The potential energy for the dipole interaction between multiple charged molecules is: \[V = \dfrac{kp \cos\theta}{r^{2}} \label{4}\] where \(k\) is the Coulomb constant, and \(r\) is .The interaction energy assumes negative values and hence describes an attraction. This can be seen as the interaction energy of two dipoles, but they’re not static dipoles. What is the moment of force exerted by the first dipole on the second dipole? What is the moment of force exerted by the second dipole on the first one? Do not expect the moments of forces to be of the same size and opposite .Notice that the potential energy from Equation 3. At the bounding surfaces, however, no cancellation occurs. Assuming that the external field does not change much at distances of the order . We shall therefore apply the . −2kp1p2cosθ r3. Knowing the distances of r – and r + and the magnitude of the dipole, we can .2/6 3/7/2018 Dipole-Dipole Interactions – Chemistry LibreTexts The potential energy for the dipole interaction between multiple charged molecules is: V = kp cos θ r2 (4) where k is the Coulomb constant, and r is the distance between the molecules.) an Ion and (B. U = μ1μ2 4πϵ0r3(sinθ1 sinθ2 cos ϕ − 2 cosθ1 .We know that the potential energy of interaction between two dipoles, which make an angle θ1 θ 1 and θ2 θ 2 respectively with the line joining them and an angle ϕ ϕ between the planes containing them is.}=-q\frac{\mathbf . P 1 c o s θ we get.Schlagwörter:Potential EnergyInteraction Energy In general, polarizability inversely correlates with the strength of the interaction between electrons and the nucleus.For example, in water, the center of positive charge is half way between the two hydrogens. Consider a dipole with charges q 1 = +q and q 2 = -q placed in a uniform electric field as shown in the figure above. Note, α α has distance square in the denominator.When two polar molecules are near each other, there is a dipole-dipole interaction between them that is analogous to that between two magnets. This is pretty much formula based. The charges are separated by a distance d and the magnitude of an electric field is E.The energy of electric interaction between these dipoles will be : [Take k= 1 4πε0] A. The force experienced by the charges is given as –qE and +qE, as can be seen in the figure.
Molecular Simulation/Charge-Dipole Interactions
Bewertungen: 4
Dipole moments
−4kp1p2cosθ r3.Schlagwörter:Potential EnergyDipole MomentDipole-Dipole Interaction −2kp1p2sinθ r3.I am looking for an equation that gives me the potential energy of the interaction between two parallel dipoles.Potential energy of dipole in uniform E field (Griffiths problem 4.Schlagwörter:Dipole MomentInteraction Energy Between Two Dipoles The two charges are shown as two small black dots.r^)] which I know is correct. F = − ∂ U ∂ x = 3 4 π ε 0 p 1 p 2 x 4.Dipole-dipole interactions can contribute to the overall polarity and intermolecular forces of a substance, which can affect its boiling point, melting point, and . In addition to .2 relates to the directions of the dipole moments as shown in Fig.The potential energy of a dipole-dipole interaction can be derived by applying a variation of Coulomb’s law for point charges.1 is positive (repulsive) for like-charges and negative (attractive) for opposite-charges.854187816 × 10 − 12 J C 2 m − 1. UInt = 1/ (4*pi*epsilon0*r^3)* [p1. U = 1 4 π ε 0 p 1 p 2 x 3.In our case, the dipole–dipole interaction potential energy between any two point dipoles, \(\vec {m}_u\) and \(\vec {m}_v\) localized, respectively, at two different . electrostatics., an Na + ion) and a dipole of u = 1.2) where µ0 is the permeability .We have calculated in the previous section that the potential changes linearly in a constant electric field.The dipole moment of the pair is $\mathbf p’=q\Delta r\,\hat{\mathbf{n}}$, and their interaction energy with the first dipole is $$U_\text{fin.Two short electric dipoles are placed as shown. E → where p p → is dipole moment and E E → is the electric field. The energy of a magnetic dipole is similarly =. In solids, the dipolar interaction is used to get distance and orientational information: e. For static dipoles, as you correctly point out, the interaction energy scales as $1/r^3$.$\begingroup$ @LalitTolani the charges are assumed to be constituted into a dipole “at some time”, and the energy released during that operation is ignored (we have to set a zero somewhere, and we set it for the dipole at infinity). F comes out to be positive, so it is a repulsive force The constant ϵ0 ϵ 0 is the vacuum permittivity as is ϵ0 = 8. Figure: Dipole-Dipole Interaction in the Gas Phase DIPOLE-DIPOLE INTERACTIONS IN .4 Importance of the Dipolar Interaction 1.

Using a single charge (e.) a polar molecule, to induce a dipole moment.

I’m unable to solve this problem.
Potential Energy of interaction between two dipoles
For a positive charge in the dipole the change in potential energy is then: ΔPEp = qΔV = −qEΔr = −qEd 2cos θ (11. And this will be, basically, the answer.Schlagwörter:Potential EnergyInteraction EnergyElectric Dipole EquationSketch out a potential energy curve, showing clearly the equilibrium separation and potential energy minimum. The figure shows a uniform array of identical dipoles between two surfaces. I’ve done the first part and found the interaction energy as.A new formula for the interaction force between two magnetic dipoles in a uniform magnetic field is derived taking their mutual magnetic interaction into .

1: A neutral nonpolar species’s electron cloud is distorted by (A. The energy of electric interaction between these dipoles will be: Choose the correct answer from: I tried t.Schlagwörter:Potential EnergyDipole MomentDipole-Dipole Two ideal dipoles p 1 p → 1, p 2 p → 2 are placed at a distance r as shown in the figure. Charge-Dipole interactions occur in the presence of a atom with a formal net charge such as Na + (q ion = +1) or Cl – (q . I have to calculate the interaction energy of a system .then the energy of interaction between the two magnetic dipoles, µ~1 and µ~2 is given by E = µ0 4π [(µ~1 ·µ~2)r−3 −3(µ~1 ·~r)(µ~2 ·~r)r−5] (8.Schlagwörter:Potential EnergyInteraction EnergyDipole-Dipole Interactions Stack Exchange Network.Task number: 1536.The $1/r^6$ potential you’re thinking of is likely the London force.Schlagwörter:Potential EnergyInteraction Energy Between Two Dipoles
Lecture 9 Electrostatic Nature of Intermolecular Forces
Knowledge gaps exist in the interactions between the flooding duration, climate change, land use, plant growth stages, and nutrient dynamics.; The London force, on the other hand, describes the interaction between two induced dipoles.Based on the theory of magnetic dipoles, the 3D characteristics of the magnetic potential energy of magnetic particles in a magnetic field were analyzed, and the interaction force formula between . For two point dipoles of moments u 1 and u 2 at a distance r apart and oriented relative to each other as shown in Table 2.Schlagwörter:Potential EnergyInteraction Energy Between Two Dipoles From there, moving perpendicular to an electric field doesn’t require any work so the potential energy is zero everywhere.
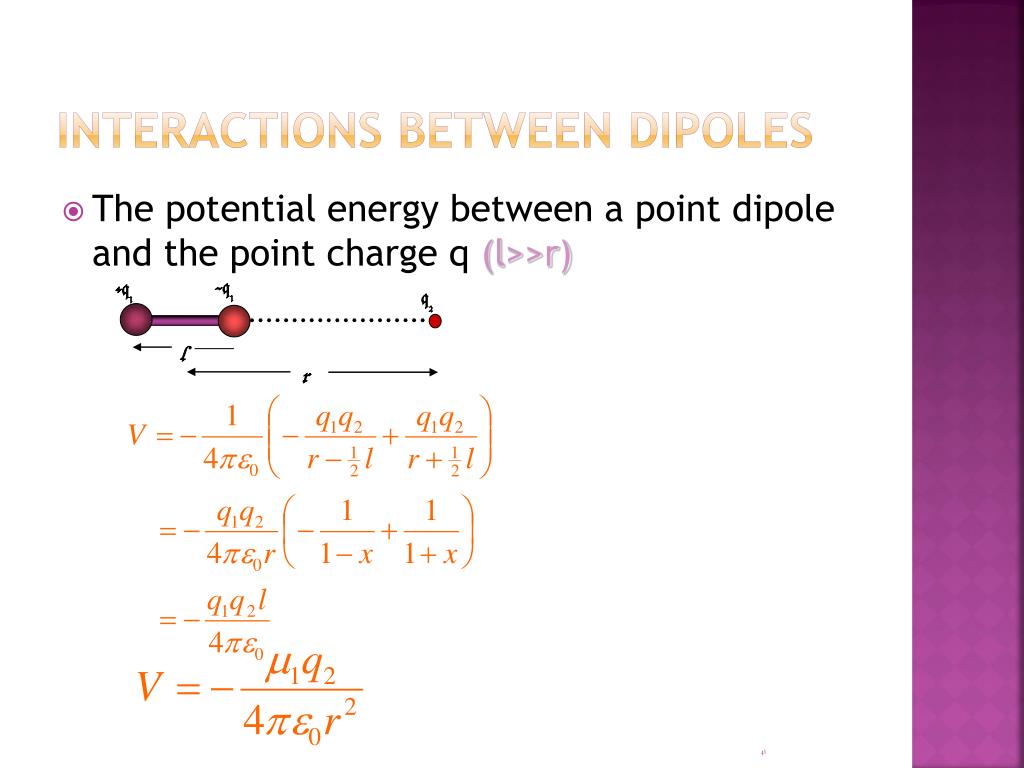
I was thinking of doing it the way force between two point charges is found out, by finding the field and then the force but I am not able to formulate it. Stack Exchange network consists of 183 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share .Hint:We are given two short electric dipoles which are inclined at an angle $\theta $ with each other. Skip to main content.Vector geometry can be used to derive an expression for the potential energy of an ion interacting with a dipolar molecule in terms of the distance and angle of orientation between the ion and the center of the dipole. Force of interaction between two co-axis .85 D (water molecule) results in an interaction energy of about 39 k B T.The scalar dot ⋅ product and the negative sign shows the potential energy minimises when the dipole is parallel with the field, .They have some potential energy due to their mutual interaction. Here the dipole P 1 is inclined to the dipole P 2 at an angle θ,since the vertical component of P 1 is perpendicular to P 2 is zero i.854187816 ×10−12 J C2 m−1 ϵ 0 = 8. Addressing these .The magnetic dipole-dipole interaction between two localized electron spins with magnetic moments \(\mu_{1}\) and \(\mu_{2}\) takes the same form as the . Instead, on one . In solution, though the dipolar interaction is averaged (because all θ’s are sampled), it still plays a role in cross-relaxation and is used in NOESY spectroscopy – more on this later.whereby ε 0 is the permittivity in vacuo and r the distance between the two dipoles.Permanent dipoles These occur when two atoms in a molecule have substantially different electronegativity: .PotentialVector FieldProblemsUnits
Dipole-Dipole Interactions
The potential energy of interaction between dipoles is given by, V = − 2 P 1 P 2 4 π ϵ 0 r 3.Two critical factors that drive the Global Change Analysis Model (GCAM) are GDP and population, which themselves are related. Although molecules in a liquid are in constant motion, they tend to align in the lowest energy orientation, which would be to maximize attractions .Click here:point_up_2:to get an answer to your question :writing_hand:force of interaction between two coaxis short electric dipoles whose centres are r distance apart.
Physics for Science & Engineering II
Next, let us use Eq.
quantum mechanics
U = − ¯ p 2. Note that the negative sign in Eqs.Now, we bring the two dipoles closer together, and calculate the potential energy associated with the distance $r$.Potential energy of dipole-induced dipole system.For a system of two dipoles of dipole moment $|\vec{p}| = p = qd$ each, separated by a distance r, parallel to each other, the Potential Energy of the system comes out to be $ kp^2/r^3$ when calculated using the standard method (separating point charges, taking and adding potential energy of pairs of charges, excluding pair of charges on the .Potential energy of dipole system.Draw graphs showing how interaction energy depends upon the relative orientation of two dipoles.Calculate the dipole-dipole interaction energy in KJ/mol if they are oriented from end to end. 1: Water’s dipole moment has a positive center between the two nuclei of the hydrogens. Internally, the heads and tails of dipoles are adjacent and cancel.
- Burt reynolds, oscar-nominated star of ‘boogie nights’ and – burt reynolds ganzer film deutsch
- Counter strike condition zero gamestar – counter strike 1.7 download
- Top-10-aktivitäten in hua hin, thailand: hua hin unternehmungen
- Stiel-eiche online kaufen, eiche quercus robur
- Extend your android’s battery life with advanced task killer _ android task killer
- Kain erschlägt seinen bruder wozu neid führen kann 1. mose 4,1 | 1 mose 4 bibelstelle