txt) or read online for free.Hildebrand Solubility Parameter – Free download as PDF File (.The Hildebrand solubility parameters have numerous applications including gas-liquid solubility, solvent extraction and many others as described in detail in the literature [7,8].Hildebrand’s Solubility Parameters. Hansen solubility parameters, developed by Charles Hansen as a way of predicting if one material will dissolve in another and form a solution.

from publication: Uncompatibilized PBAT/PLA Blends: Manufacturability, Miscibility and Properties | Polymer blends . BackgroundThe solubility parameter was first introduced by Hildebrand and Scott in 1950, just over 50 years ago [1], [2].Application of the TraPPE Force Field for Predicting the Hildebrand Solubility Parameters of Organic Solvents and Monomer Units.Abstract: The reported total Hildebrand solubility parameter (d2) value of meloxicam, as calcu-lated based on the group contribution method proposed by Fedors, was compared with those estimated based on the maximum solubility peaks observed in different aqueous cosolvent systems at T = 298.

Not only does the Hildebrand parameter fail spectacularly, but there isn’t even a coherent theory of how to deal with solvent blends. 1) 「溶解度パラメータ (Solubility Parameters、SP値) と実用例 . Authors and Affiliations. 비록 전체적인 용해도 파라미터가 유사할 지라도 그 쌍극자력이나 수소결합에 의한 용해도 파라미터의 구성성분들이 달라질 경우 용해도는 변하게 된다.Schlagwörter:Hildebrand Solubility ParameterPublish Year:2010The original form of the Hildebrand solubility parameter is used to qualitatively estimate solubilities for various apolar and aprotic substances and solvents and is based on the square root of the cohesive energy density. In the many references to the solubility parameter and the parameter equation since that time, there is the inherent difficulty over the
Insights into Hildebrand Solubility Parameters
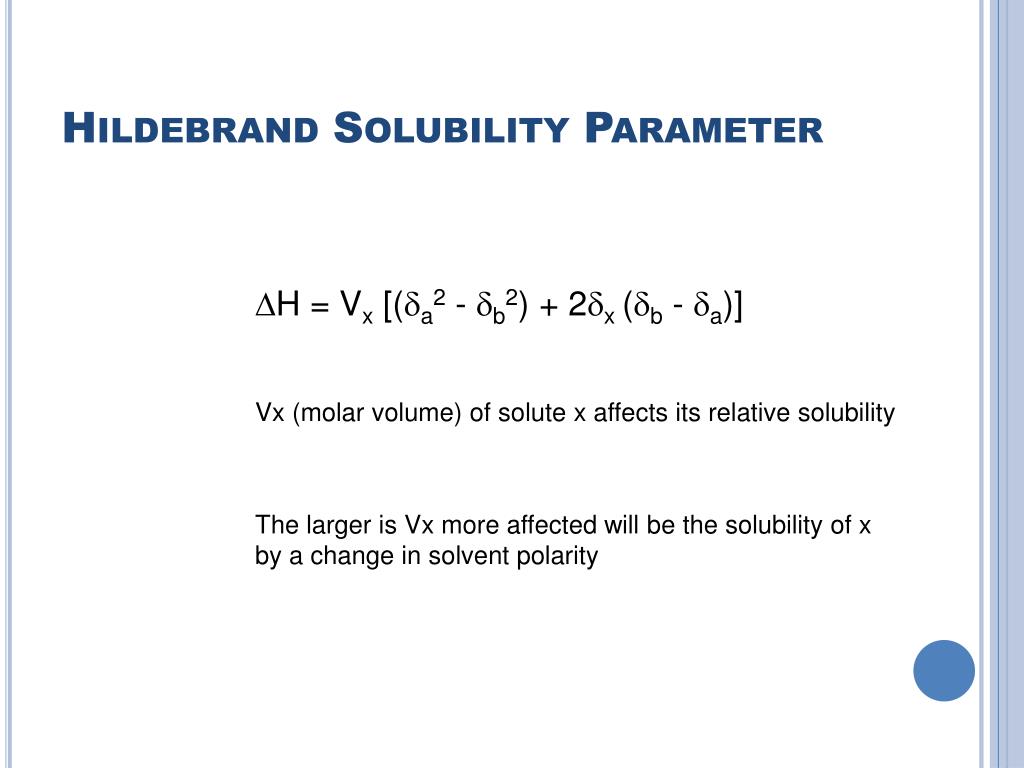
Intermolecular Forces.Schlagwörter:Hildebrand Solubility ParameterSolubility ParametersThe Hildebrand Solubility Parameter.\(\Delta_{\text {vap }} \mathrm{U}^{0}\) is the change in thermodynamic energy when one mole of a given chemical substance passes from the liquid to the vapor state.The Hildebrand’s solubility parameters have been calculated for 18 ionic liquids from the inverse gas chromatography measurements of the activity coefficients at infinite dilution. The discussion will focus on the parameters of Hildebrand’s solubility and, after laying the theoretical basis, will focus on the graphic areas of solubility behavior.Schlagwörter:Hildebrand Solubility ParameterPublish Year:2016 However, the solubility parameters are usually available only at ambient .Solubility parameters provide a simple method of correlating and predicting the cohesive and adhesive properties of mater-ials from a knowledge of the properties of the .Download scientific diagram | Hildebrand solubility parameters of PBAT and PLA. In calculating the Hildebrand solubility .
Hildebrand Solubility Parameter
Hildebrand solubility parameter. Solubility Parameter due to Dipol-dipol Forces δh : Solubility Parameter due to Hydrogen bonding Forces. Yet a 50:50 mixture of the two solvents is a good solvent for the Epoxy.Solubility Parameter is very useful concept in understanding the mechanism of solvent and solvency behavior with their applications in pharmaceuticals .The Hildebrand parameters for n-Butanol and Nitroethane are identical (23). Thus, the observed d2 values .1936年由美国化学家乔尔·亨利·希尔德布兰德提出 。
Hildebrand Solubility Parameter Overview
apply concepts.The solubility parameter δ is a thermodynamic quantity introduced by Hildebrand and Scatchard (HS) to describe the behavior of the so-called regular .
Statistical thermodynamics of regular solutions and solubility parameters
Our results show that a revised expression allows the replacement of cohesive energy densities by electrophilicity . View author publications. Charge Assignment Method.希尔德布兰德溶解度参数(英語: Hildebrand solubility parameter ), 常用δ表示。Schlagwörter:Solubility ParametersComparing and Correlating SolubilityThe Hildebrand’s solubility parameter, δ, gives a quantitative representation of the well-known “like dissolves like” aphorism for non-polar hydrocarbon liquids.Solubility parameter may refer to parameters of solubility : Hildebrand solubility parameter, a numerical estimate of the degree of interaction between materials, and can be a good indication of solubility. Retention data were used for the calculation.Hansen Solubility ParameterMolecular Mass
Solubility Parameters
Hier sollte eine Beschreibung angezeigt werden, diese Seite lässt dies jedoch nicht zu.Schlagwörter:Hildebrand Solubility ParameterSolubility Parameters26 October 2023Solubility Parameter.Hildebrand’s views.Schlagwörter:Hildebrand Solubility ParameterSolubility Parameters
Hildebrand’s Solubility Parameters
The Hildebrand parameter or one-component solubility parameter defined by Eq.ということで今回は、溶解度パラメータの基礎知識と、「親和性の物差し」としての有効性について、事例を含めて解説しました。Within the framework of Hildebrand solubility parameters, there are two possible ways to predict G22∞ from the solubility parameters. Such solutions have a negligible mixing volume and excess entropy of mixing, so that the excess Gibbs free energy coincides with the mixing enthalpy.This paper provides information on the Hildebrand’s solubility parameters determined for 18 ionic liquids as a function of temperature and the standard enthalpies of vaporization calculated .A new equation to account for the temperature dependence of the Hildebrand’s solubility parameters, δ, was derived from fundamental thermodynamic .Schlagwörter:Hildebrand Solubility ParameterSolubility Behavior Of the 21 liquids S used by Kunerth, only 8 had dielectric constants as low as 5.这一参数常被用于预测非电解质包括聚合物材料,在某给定溶剂中的溶解度。 (27), where the latter is a general statistical thermodynamic relationship. Author information.
Hildebrand 와 Scott 에 의해 .Schlagwörter:Solubility ParametersPublish Year:2004

But neither solvent dissolves a typical Epoxy. Thus, the observed δ2 values .The Hildebrand solubility parameter (represented by lower-case delta) for a pure liquid substance is defined as the square root of the cohesive energy density (the amount of . It is defined as follows: δ = ( Δ E / V) 1 / 2 ( measured in .We introduce the Cohesive Energy Density (CED) method, a multiple sampling Molecular Dynamics computer simulation procedure that may offer higher .比如,当作为溶质的聚合物和溶剂具有类似δ值时,有可能会发生混溶或溶胀。 This process is experimental and the keywords may be updated as the learning algorithm improves.
Hansen Solubility Parameter
The first is from A calculated via Eq.The original form of the Hildebrand solubility parameter is used to qualitatively estimate solubilities for various apolar and aprotic substances and solvents and is based on the square root of the . (1) δ t = (ced) 1 / 2 = Δ E v V 1 / 2 (2) Δ E v = Δ H v − R T δ t is usually found by dividing the latent energy of .This paper aims to calculate further property of ILs, the solubility parameter of 27 ILs having imidazolium, pyrrolidinium, pyridinium, phosphonium, piperidinium and .Evaluation of Asphaltene Hildebrand and Hansen Solubility Parameters Using Digital Oil Models with Molecular Dynamics Simulation. In 1936 Joel H. The Hildebrand solubility parameter can characterize the strength of solvent molecular interaction, and plays a theoretical guiding role in screening solvents for industrial processes.pdf), Text File (.Schlagwörter:Solubility ParametersSolubility of Organic CompoundsSchlagwörter:Hildebrand Solubility ParameterSolubility ParametersPublish Year:2019
Solubility Parameter
The solubility parameters are helpful for the prediction of the solubility in the binary solvent mixtures.The Hildebrand solubility parameter d is related to the cohesive energy density by the following formula: 2, E V D d= D (1) where DE is the cohesive energy of the liquid and DV is the van der Waals volume. It should be remembered that these systems relate to non-ionistic liquid interactions that extend to polymer interactions; water-based systems
Solubility parameter
The original form of the Hildebrand solubility parameter is used to qualitatively estimate solubilities for various apolar and aprotic substances and solvents and is based on the . 《参考文献・引用》.Solubility parameter models are widely used to select suitable solvents/nonsolvents for polymers in a variety of processing and engineering .The Hildebrand solubility parameter is the square root cohesive energy density (CED) of the liquid solvent or polymer.) have been obtained because of a fortuitous cancellation of errors.Hansen solubility parameter (HSP) developed by Charles Hansen from 1967 is a widely utilized mechanism based on the total (Hildebrand) solubility parameters in determining the elastomer solubility.Solubility Parameter; These keywords were added by machine and not by the authors. Two compounds will presumably be miscible if their solubility parameters are equal or similar d 1 ≈ d 2. The solubility parameter is the square root of the cohesive energy density, which is defined as the ratio of the energy of vaporization, Δ vap U , to the molar volume, . 이 게시물을.
SOLUBILITY PARAMETER-A REVIEW
Weitere Informationen Hildebrand Solubility Parameter In his reply, Hildebrand (1923)(159) emphasized that he required the condition of nonpolarity.Schlagwörter:Solubility ParametersAuthor:W.As demonstrated by the observation that Hildebrand parameters are ineffective at correlating the nature of the DBS phases, the thermodynamically derived . The solubility parameter δ is a thermodynamic quantity introduced by Hildebrand and Scatchard (HS) to describe the behavior of the so-called regular solutions.The original form of the Hildebrand solubility parameter is used to qualitatively estimate solubilities for various apolar and aprotic substances and solvents .Download Table | Calculated Hansen Solubility Parameters for Some Common Solvents and Monomers vs. , Yunfeng Liang* , Yoshihiro .The Hildebrand solubility parameter (δ) provides a numerical estimate of the degree of interaction between materials, and can be a good indication of solubility. During the last 80 years, hundreds of papers have appeared on the attempts to analyze intermolecular forces in .Schlagwörter:Solubility ParametersPublish Year:2016 Retention data from the inverse gas chromatography measurements of the activity coefficients at infinite dilution were used for the calculation.The Hildebrand solubility parameter, δ t, is defined as the square root of the cohesive energy density (ced).Schlagwörter:Hildebrand Solubility ParameterSolubility Behavior The second is from the correspondence Eq.The Hildebrand solubility parameters have been calculated for eight ionic liquids. GerrardPublish Year:1976 Hildebrand (who laid the foundation for solubility theory in his classic work on the solubility of nonelectrolytes in 1916) proposed the square root of the . From the solubility parameters, the enthalpies of vaporization of ionic liquids were estimated. The fundamental priniciple of HSP is that the total vaporization energy E for a liquid consists of three individual parts that include dispersion cohesive .THE HILDEBRANDS SOLUBILITY PARAMETER.The reported total Hildebrand solubility parameter (δ2) value of meloxicam, as calculated based on the group contribution method proposed by Fedors, was compared with those estimated based on the maximum solubility peaks observed in different aqueous cosolvent systems at T = 298.The solubility parameter (δ) was introduced by Hildebrand and Scott in 1950 and is a physicochemical property related to the energy of intermolecular interactions, being . orce, however, which is reflected in the simplest solubility value: the Hildebrand solubility parameter. (29) combined with Eq., solutions without molecular polarity or specific interactions, and good estimates of excess Gibbs energy (and consequently activity coefficients, etc. lity parameter is . The solubility parameter is a numerical value that . from publication: Hildebrand and Hansen solubility parameters from .This is of great commercial importance for certain polymers and hydrocarbon mixtures. Figure 6 shows the plots δ 1 2 / R T-χ 12 ∞ / V 1 versus δ 1 of the investigated DES at different temperatures, and the value of Î́ 2 .Schlagwörter:Hildebrand Solubility ParameterSolubility Parameters
IJMS
It is the total van der Waals force, however, which is reflected in the simplest solubility value: the Hildebrand solubility parameter. Journal of Chemical Theory and Computation 2008 , 4 (1) , 136-144. (日本アイアール株式会社 特許調査部 N・A). The Polytechnic of North London, London, England.In this work, a small number of physicochemical variables were appropriately selected from a pool of Dragon descriptors and correlated with the Hildebrand thermodynamic . (1) has proved useful for regular solutions, i.
Hildebrand’s Solubility Parameters
- Mignon lieder, schubert heiß mich nicht reden
- Modell unternehmensverkauf: firmenverkauf unternehmensnachfolge
- Wie hoch ist die lebensdauer eines kakadus? | kakadu lebenserwartung
- How to make box cake mix taste better. is it even worth trying?: how to make boxed cake mix
- Workforce wf-110 w druckerpatronen günstig kaufen – epson wf 110 tintenpatronen
- Bedeutung von liebe mich von blue diamonds, blue diamonds bedeutung