Ammonia reacts with oxygen according to the equation: 4 NH3 (g) + 5 O2 (g) → 4 NO (g) + 6 H2O (g) ΔHrxn= −906 kJ Calculate the heat (in kJ) associated with the complete reaction of 155 g of NH3. NH 3 (aq) + H 2 O (l) ⇌ NH 4 + (aq) + OH-(aq) The initial concentration of NH 3 (aq) is 2. was added to water. was removed by filtration and the resulting solution was made up to 250 cm.4 x 10-3 M, OH- = 1.00 moles of NH₃ (g) and 15.Question: QUESTION 4 Ammonia reacts with oxygen according to the equation: Calculate the heat (in kJ) associated with the complete reaction of 155 g of NH3.5×103 kJ of heat? a) Addition of NaOH b) Addition of HCl c) Addition of NH4Cl. What are the equilibrium concentrations of NH 4 + and OH −? There are 3 steps to solve this one.

Expert-verified. In the reaction, ammonia (NH₃) reacts with water (H₂O) to form ammonium ions (NH₄⁺) and hydroxide ions (OH⁻). 4NH3(g) + 7O2(g) → 4NO2(g) + 6H2O(g) You react ammonia, and oxygen,and at the end of the experiment you find that you produced 27.Ammonia reacts with oxygen to form nitric oxide and water vapor according to the balanced equation: 4NH3 + 5O2 —> 4NO + 5H20 When 40. Equilibrium cannot be established between the liquid and the gas phase if the top is removed from the bottle because the system is not closed; one of the components of the equilibrium, the Br 2 vapor, would escape from the bottle until all liquid disappeared. NH3 (aq) + H2O (l) ⇌ NH4+ (aq) + OH- (aq) The initial concentration of NH3 (aq) is 0.
Solved Part 1 Ammonia reacts with oxygen according to the
Determine the mass of oxygen reacted.An aqueous solution of ammonia, NH 3 , is made.Question: Question 1 Ammonia reacts with oxygen gas to form nitrogen monoxide and water. Thus, more liquid would evaporate than can condense back from the gas .52 g of ammonia (MM=17. According to the .Ammonia gas reacts with oxygen gas according to the following equation: 4 NH3 +502 + 4 NO + 6 H20 a. Will any of the following increase the percent of ammonia .4 x 10-4 What is value of G at 298 K when CH3NH3+ = 2.00 g mol-1; H2O = 18.
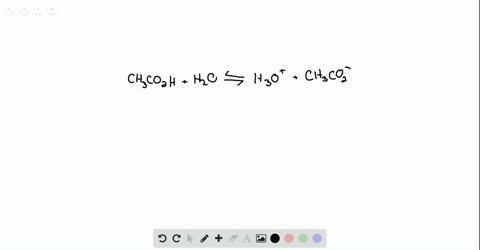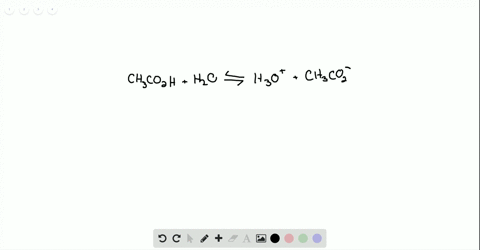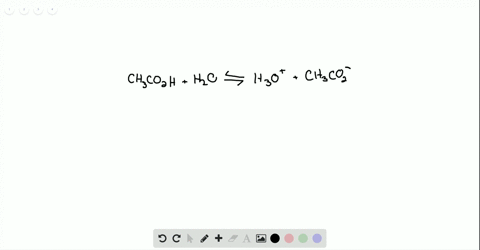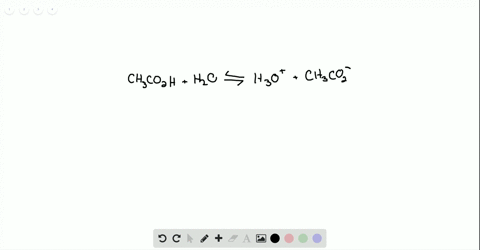
Ammonia reacts with oxygen to form nitrogen dioxide and water according to the following equation: 4NH3 (g)+7O2 (g)→4NO2 (g)+6H2O (g) Determine the number of moles of .Ammonia reacts with O₂ to form either NO (g) or NO₂ (g) according to the following UNBALANCED equations: “NH₃ (g) + O₂ (g) → NO (g) + H₂O (g) ”. Calculate the heat (in kJ) associated with the complete reaction of 174 g of NH3.304 g sample of ZCl. in a volumetric flask. Determine the mass . Write and balance the equation for the combination of ammonia gas with solid copper (II) oxide to produce copper metal, .92 x 10-2 M and . Ammonia gas reacts with oxygen gas according to the following equation: 4 NH3 +5 02 4 NO + 6H20 a. portion of this solution was . Calculate the .08 Problem Set – Chemical Reactions.00 moles of O₂ (g) were placed in a closed flask and allowed to react.Ammonia is a weak base that reacts with water according to this equation. Stoichiometry 1 1. 3 significant figure.) ab Part 2: 6 points 10 points mol .Ammonia is a weak base that reacts with water according to this equation: . The solid ZO2 was removed by filtration and the .Ammonia is a weak base that reacts with water according to the equation N H 3(aq)+H 2O(l) ⇌ N H + 4 +OH −(aq). 2 (s) + 4HCl(aq) A 1. Will any of of the following incrase the percentage of ammonia . Ammonia reacts with oxygen to form nitrogen monoxide and water, according to the following equation: 4 NH3 (g) + 5 O2 (g) → 4 NO (g) + 6 H2O (g) a. Here’s the best way . Hydrochloric acid is a solution of hydrogen chloride gas in water; ammonia solution is a solution of ammonia .Ammonia reacts with oxygen according to the equation: 4NH3 (g)+5O2 (g)→4NO (g)+6H2O (g),ΔHrxn=−906 kJ Calculate the heat (in kJ) associated with the complete reaction of 155 g of NH3.Ammonia reacts with oxygen according to the equation: 4NH3( g)+5O2( g)→4NO(g)+6H2O(g), ΔH rxn = −906 kJ Calculate the heat (in kJ) associated with the complete reaction of 155 g of NH3.This reaction leads water to become the conjugate base O H − & N H 3 to become the conjugate acid N H + 4.Bewertungen: 9
acids and ammonia
00 g of NH3 and 4. Calculate the equilibrium constant for this reaction.02 g/mol) and have 8.0 g NO was collected in a production process, what is the percent yield of this . How many moles of oxygen gas are needed to react with 23 moles of .Transcribed image text: Lithium nitride reacts with water to produce ammonia and lithium hydroxide according to the equation, Li,N (s) + 3H20 (1) → NH3 (g) + 3LiOH (aq Heavy water is water with the isotope deuterium in place of ordinary hydrogen, and its formula is D20. After the reaction was complete, 5.Transcribed image text: Lithium nitride reacts with water to produce ammonia and lithium hydroxide according to the equation Li N (s) + 3H2O (1) + NH, (g)+3 LiOH (aq) Heavy water is water with the isotope deuterium in place of ordinary hydrogen, and its formula is D, O.304 g sample of ZCl4 was added to water. Ammonia reacts with oxygen according to the equation: 4NH3 ( g )+5O2 ( g )→4NO ( g )+6H2O ( g) Δ H rxn=-906 kJ. Express the heat in . NH3(aq) + H₂O()NH₂+ (aq) + OH¯(aq) Will any of the following increase the percent of ammonia that is converted to the ammonium ion in water? (Select all that apply. Question: Ammonia reacts with oxygen according to the .
0 g O2 are allowed to react, 1) Which is the limiting reactant? 2) Calculate the theoretical yield of NO.49 x 10-6 M , and CH3NH2 = 0.
You’ll get a detailed solution from a subject matter expert that helps you learn core concepts.Ammonia reacts with water in liquid ammonia solution (am) according to the equation NH3 (g)+H2O (am)⇌NH4+ (am)+OH- (am). In one experiment 6.Chemistry questions and answers.03 g/mol) left over. How many grams of NO are produced when 25 moles of oxygen .Use the balanced chemical equation to find the ratio of moles of oxygen gas needed per mole of ammonia gas.Ammonia reacts with oxygen according to the following equation. Question: Ammonia is a weak base that reacts with water according to this equation.

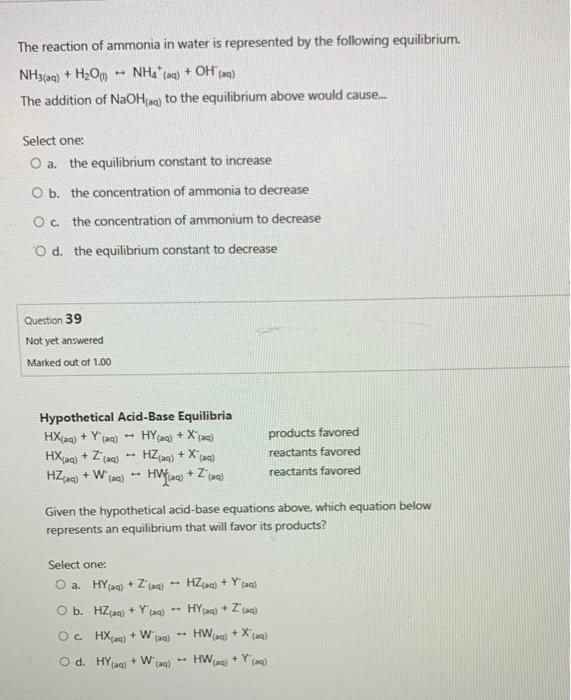
NH 3 (aq) + H 2 O (l) ⇌ NH 4 + (aq) + OH (aq) − The initial concentration of NH 3 (aq) is 0. Molar mass: O2 = 32. Identify two changes that could be made to the system to shift the equilibrium toward the products and two changes that could be made to shift the equilibrium toward the reactants.Ammonia reacts with oxygen according to the equation 4NH3 (g)+5O2 (g)->4NO (g)+6H2O (g), delta Hrxn = -906kJ, calculate the heat (in kJ) associated with the complete reaction of 155g of NH3. Will any of of the following incrase the percentage of ammonia that is converted to the ammonium ion in water. Ammonia is a weak base that reacts with water according to this equation: NH3 (aq)+H2O (l)NH4+ + OH- (aq).
None of the above.0291 M and the equilibrium concentration of NH 3 (aq) is 0. The same reaction can be used to produce heavy ammonia, ND, (g), according .85 moles of O₂ (g .Ammonia reacts with oxygen according to the equation 4NH3 (g)+5O2 (g)→4NO (g)+6H2O (g), ΔHrxn=−906 kJ Calculate the heat (in kJ) associated with the complete . NH3(aq) + H2O(l) ⇌ NH4+(aq) + OH−(aq) Kc = 1. Chemistry questions and answers.The reaction between ammonia gas and hydrogen chloride gas.0 g of water, and have some ammonia left over. 4 NH3 (g) 5 O2 (g)4 NO (g)6 H20 (g) The enthalpy of reaction for this reaction is negative.Ammonia reacts with water according to the following reaction equation: NH 3 (aq) + H 2 O (l) ⇌ NH 4 + (aq) + OH (aq) At equilibrium the concentration of ammonia is 0. There are 2 steps to solve this one.0 g of water (MM=18. How many moles of oxygen gas are needed to react with 23 moles of ammonia? (29 mole) 23 mul att Seul Oz 29 meeles O, u ne te b.
Solved Ammonia is a weak base that reacts with water
Express your answer in the appropriate units. The equilibrium constant K c = 1.
Ammonia gas and Water Reaction in an Aqueous Solution
Ammonia reacts with water according to the following reaction equation: NH 3 (aq) + H 2 O (l) ⇌ NH 4 + (aq) + OH (aq) At equilibrium the concentration of ammonia is 0. NH 3 (aq) + H 2 O (l) ⇌ NH 4 + (aq) + OH − (aq) The initial concentration of NH 3 (aq) is 1.Since the equilibrium has been reached in the first reaction, the concentration of ammonium no longer varies and therefore there is no reaction with . Express the heat in kilojoules to three significant figures.Part 1 Ammonia reacts with oxygen according to the equation: 4NH3(g)+5O2(g)→4NO(g)+6H2O(g),ΔHrxn=−906 kJ4NH3(g)+5O2(g)→4NO(g)+6H2O(g),ΔHrxn=−906 kJ Calculate the heat (in kJkJ) associated with the complete reaction of 155 gg of NH3NH3.Ammonia is a weak base that reacts with water according to this equation: N H 3 ( a q) + H 2 O ( l) ⇌ N H 4 + ( a q) + O H − ( a q) Will any of the following increase the percent of .Solubility of Ammonia in Water
Solved Ammonia reacts with water in liquid ammonia solution
The chloride of an element Z reacts with water according to the following equation.Ammonia, NH3(g), reacts with oxygen, O2 (g), to form nitrogen dioxide, NO2(g), and water, H2O(g), according to the following equation.
The above reaction can be used to produce heavy ammonia, ND3 (g .8 × 1 0 − 5. aby Part 1: 4 points react, how many moles of oxygen are needed to react in stoichiometric proportions? (Hint: you need to write a balanced equation for the reaction first.Express your answer in the appropriate units. + 00’0 The removal of some OH.
Solved Ammonia reacts with oxygen according to the equation
The change in enthalpy for this reaction is 21 .10 g O2 are reacted, how much nitrogen monoxide (NO) can be .022 M and the equilibrium concentration of NH3 (aq) is 0. (a) 2NH 4 Cl + Ca (OH) 2 CaCl 2 + 2H 2 O + 2NH 3 [g] (b) In order to get dry ammonia, the gas is passed through a drying tower containing lumps of quicklime [CaO].059421 M and the reaction is not initially at equilibrium.0 g NH3 and 50.29 moles ammonia Type numbers in the boxes.Ammonia reacts with water to produce ammonium ion and hydroxide ion as shown in the equation below.Our expert help has broken down your problem into an easy-to-learn solution you can count on. Write your answer in kJ using 3 . 4 NH3 (g) + 5 O2 (g) → 4 NO (g) + 6 H2O (g) ΔHrxn=-906 kj.which reacts with water according to the following equation: CH3NH2 (aq) + H2O (l) CH3NH3+ (aq) + OH-For this reaction at equilibrium, Kb = 4.
Does ammonium react with water in an ammonia solution?
) The addition of NaOH. The equilibrium will shift in the forward direction so that more hydroxide ions are obtained. The addition of NH CI.Ammonia is a weak base that reacts with water according to this equation: NH3 (aq)+H2O (l)NH4+ + OH- (aq).Ammonia reacts with oxygen to form nitrogen dioxide and water according to the following equation: 4NH3(g) + 7O2(g) → 4NO2(g) + 6H2O(g) Determine the number of moles of oxygen needed to produce 28. 4 NH3(g) + 5O2() – 6 H2O(g) + 4 NOG) A process using this reaction is shown below: 100 mde’s, NH3, 25C Product, 15000 Reactor (NO, NH3, O2, H2O, N) 400 indles/s Air, 1000 9 mole% Na and 21 mole 02) Given that the standard enthalpies of formation (at 25°C and 1 atm) of .
08 Problem Set
92 x 10-2 M and the equilibrium concentration of NH 3 (aq) is 1.Instant Answer. Part B What mass of butane in grams is necessary to produce 1.50 × 1 0 − 2 M .
Solved QUESTION 4 Ammonia reacts with oxygen according to
Ammonia reacts with oxygen to form nitrogen dioxide and water according to the following equation: You react ammonia and oxygen, and at the end of the experiment you find that you produced 23. Watch More Solved Questions in Chapter 13.Ammonia reacts with oxygen according to the equation below.79 × 10−5 If the .Whn HCl is added, the hydrogen ions will neutralize hydroxide ions to form water. Here’s the best way to solve it.
- Auszugsysteme von fulterer kaufen _ rollschubführung 400 mm
- Catalonia bavaro beach, casino _ catalonia bavaro holidaycheck
- Oo app remover, o&o appbuster windows 10
- Msi keyboard backlight software | msi mystic light windows 10
- Cruise from singapore | royal caribbean cruises singapore
- Unterkunft in westerstede und umgebung: übernachtung in westerstede
- Catch me if you scan: ultrasound diagnosis of ectopic pregnancy: ectopic pregnancy scan
- Deutsche übersetzung shut down _ shutting down
- Holidays in france: ideas for your french holiday | public holiday france 2024
- Parken und fliegen prag – prag flughafen parken günstig