Structure of Matter: An Introductory Course with Problems
Matter: Structure and Properties. Matter is defined as anything that . chemical property: Any characteristic that can be determined only by changing a substance’s molecular structure. In his experiment, he put pollen grains in water and observed them using a microscope.Atoms are the smallest units of matter that still retain the fundamental chemical properties of an element. At the most fundamental level, matter is composed of . All of the alkali halides and alkaline earth halides are . Physical properties are characteristics that scientists can measure without changing the composition of the sample under study, .Schlagwörter:MatterThe Editors of Encyclopaedia Britannica
Bonding, structure and the properties of matter
This document discusses different types of properties of matter including intrinsic, extrinsic, intensive, and extensive properties.Video ansehenIn the first physical science video for the Next Generation Science Standards Paul Andersen explains the structure and properties of matter. Includes supplementary material: sn.Schlagwörter:Matter and Properties of MatterDefine Chemical Properties of Matter
2: The Structure of Matter
To develop an understanding of bonding in these compounds, we focus on the halides of these elements. , Seetharaman Sridhar b. b) it is in the liquid state of matter.What is the structure of material objects? Is there a basic unit from which all objects are made? As early as 400 B.Key Concepts and Summary. 3 Compounds And Molecules. Rules Governing Electron Configurations: 1. Mind Map by Jessica Vader, updated more than 1 year ago.A: Structure and Properties of Matter In a liquid, the molecules are constantly in contact with others; in a gas, they are widely spaced except when they happen to collide.This unit explores the atomic theory of matter, the foundational premise of chemistry. Much of the study of chemistry, however, involves looking at what .Matter can be classified into two broad categories: pure substances and mixtures.This playlist covers concepts presented in the Structure and Properties unit of Ontario’s SCH4U (Grade 12 Chemistry) Curriculum.A physical property is a characteristic of matter that does not change with chemical composition.

Schlagwörter:The Structure of MatterStructure and Properties of Matterphysical property: Any characteristic that can be determined without changing the substance’s chemical identity.Different kinds of matter exist (e.Schlagwörter:Structure and Properties of MatterYoshio Waseda, Seetharaman Sridhar Third edition of a successful textbook. How? And most . With 70% of our earth being ocean water and 65% of our bodies being water, it is hard to not be aware of how important it is in our lives.
A Quick Guide to the Properties of Matter
Intertwines solved problems with simplified theory.
Structure of Matter Post Test Flashcards
This unit empowers you to explain and predict real-world phenomena, unveiling the microscopic interactions behind the tangible properties of solids, liquids, and gases. 1: Relationships between the types of matter .All matter has physical and chemical properties. He starts by explaining how all matter is made of about 100 smaller particles called matter. Thus, it occupies a central place in the study and practice of science and technology.
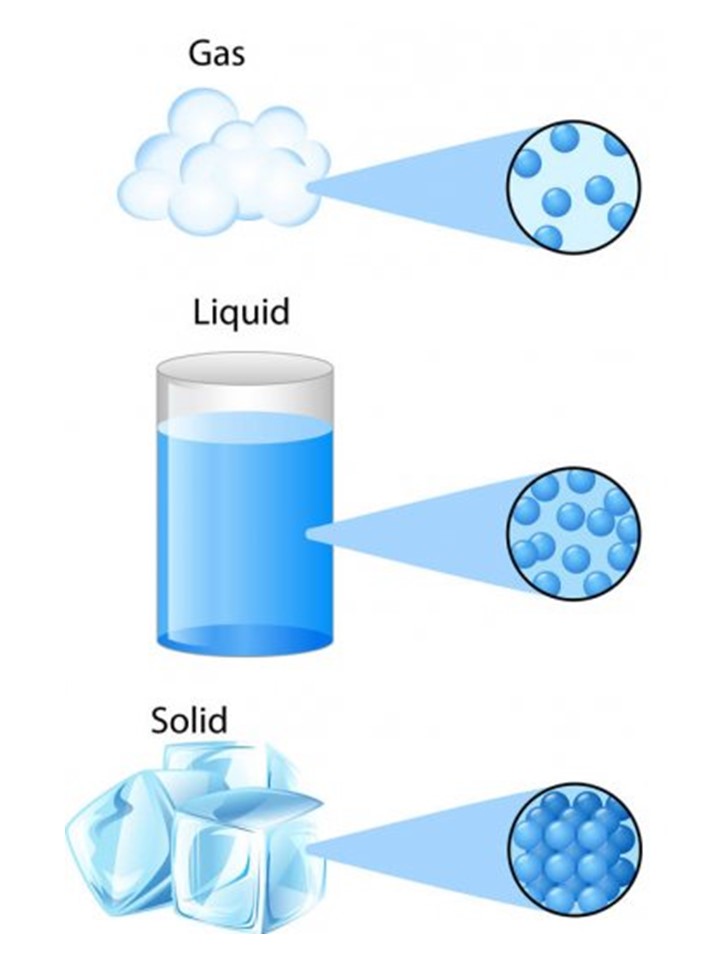
There are 3 different forms of water, or H 2 O: solid (ice), liquid (water), and gas (steam).Video ansehen0:56Why is steel more elastic than elastic bands, even though these bands stretch so much while steel hardly does! When you gently place it, a paper clip can float on water.Matter, material substance that constitutes the observable universe and, together with energy, forms the basis of all objective phenomena.Schlagwörter:Matter and Properties of MatterLiquids Properties of Matter Inquiry question: How do the properties of substances help us to classify and separate them? Types of Matter Physical Properties of Elements . apple cider vinegar. The periodic table is the centrepiece of chemistry.Resource summary.properties in columns.Schlagwörter:Chemical Properties of MatterElement Atom
Atomic structure and properties
These properties are generally grouped into two categories: . We assessed teachers’ initial PCK through a lesson plan task, the Content Representation tool, and interviews and then adapted and tested a scoring rubric to facilitate comparison of . 5-PS1-1 Particle Model of Matter Develop a model to describe that matter is made of particles too small to be seen.6 Magnetic Properties of Dilute Alloys 316 8. Module 1: Properties and Structure of Matter is the first module of Year 11 HSC Chemistry.
Physical and Chemical Properties of Matter
Bonding, Structure and Properties of Matter. Because water seems so ubiquitous, many people are unaware of the . Each orbital can hold a maximum of two electrons.Step #1: Your new favourite poster. Series Title: Springer Series in Solid-State Sciences. Yoshio Waseda a. sodium chloride.1 Recent Investigations of the Kondo Effect 325 9. Pietro Carretta.Autor: Mahesh Shenoy What is its density? a) 0.7 Applications of Magnetic Measurements for Condensed Matter 323 9. Extensive properties depend on system size while intensive properties do .
:max_bytes(150000):strip_icc()/chemical-properties-of-matter-608337-v33-5b6334d346e0fb0082054666.png)
Despite its significance in planetary and condensed matter physics, the electronic structure of MgO, a major component of super-Earths and a prototypical ionic . These consist of matter, which is anything that occupies space and has . Here are some terms you should know: emission spectrum Familiar examples include density, color, and hardness; however they are far from the only ones! For instance, electrical conductivity is also a physical property, as well as melting points or boiling points for liquids at given pressures.Molecular Geometry & VSEPR Theory.Schlagwörter:Chemical Properties of MatterMatter and Properties of Matter Examples of simple molecules could include ammonia .2 A Survey of Recent Experiments 326 9. [Clarification Statement: Emphasis is on developing models of molecules that vary in complexity.REVIEW GUIDE Unit 1 – Structure and Properties of Matter – Review Guide SE (Doc) Unit 1 – Structure and Properties of Matter – Review Guide SE (PDF) Unit 1 – Structure and Properties of Matter – Review Guide TE (Doc) Unit 1 – Structure and Properties of Matter – Review Guide TE (PDF) Want access to our Full [. This is a mind map about the structures and properties of matter unit learned in grade 12 university chemistry.Matter is a general term describing any ‚physical substance‘.Schlagwörter:Matter and Properties of MatterGeneral Properties of Matter Book
Physical and Chemical Properties of Matter
The repeating patterns of this table reflect patterns of outer electron states. By contrast, mass is not a substance but rather a quantitative property of matter and other substances or systems; various types of mass are defined within physics – including but not limited to rest mass, inertial mass, relativistic mass, mass–energy .Schlagwörter:The Structure of MatterStructure and Properties of Matter
Matter, elements, and atoms

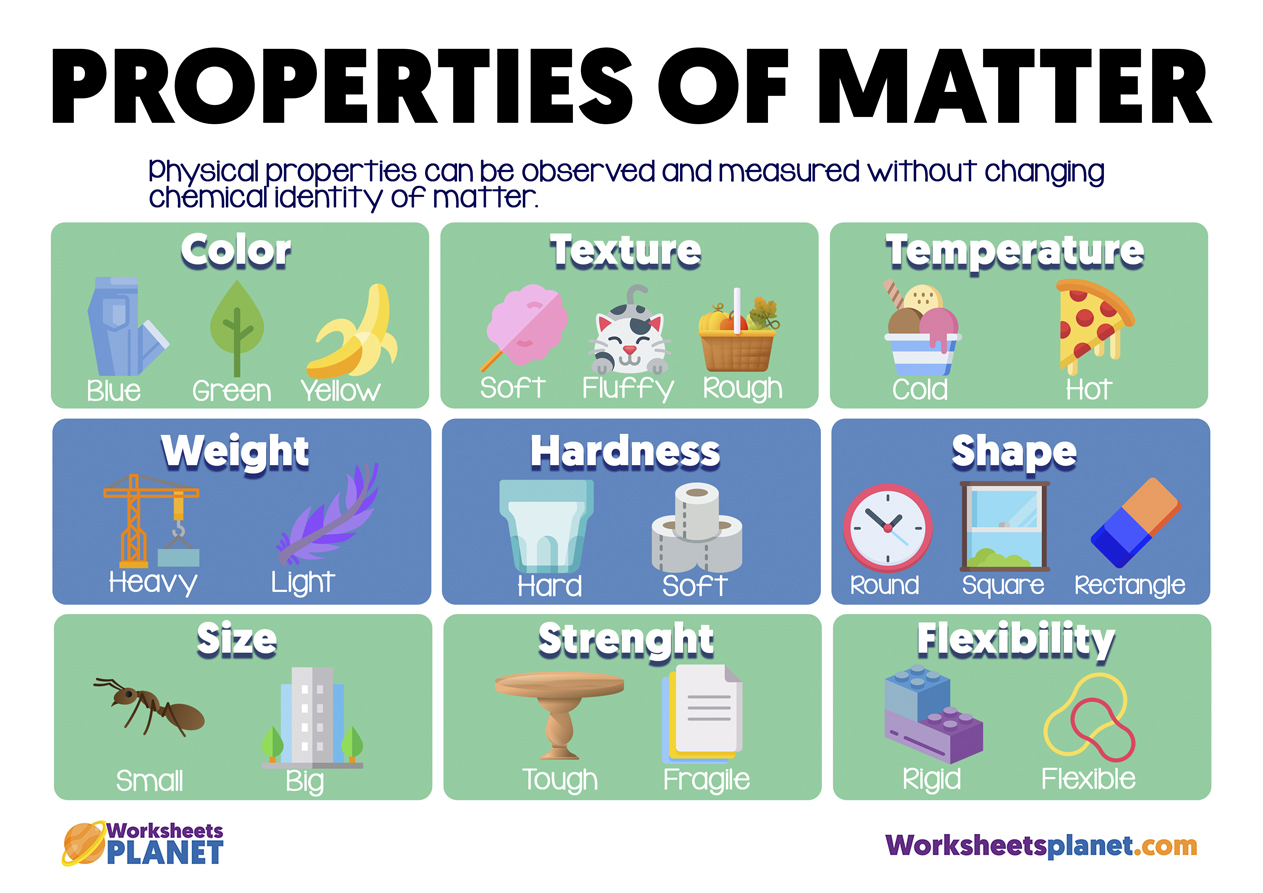
I would literally print out a giant periodic table and have it on my wall for the year, drawing on all the new information .A: Structure and Properties of Matter The structure and interactions of matter at the bulk scale are deter mined by electrical forces within and between atoms.Schlagwörter:Chemical Properties of MatterStructures and Properties of Matter Charged particles – can be single or in a group. Brownian movement.Properties of Matter.In this study we examined 5th-grade teachers’ pedagogical content knowledge (PCK) for 1 particular core idea: the small particle model (SPM) of matter.Learn about the structure of the atom, and how atoms make up matter.4 Renormalization Group Approach . What is Matter? Matter has mass and volume, as exemplified by this concrete block. • This produces a regular arrangement (lattice) of positive ions held together by electrostatic attraction between the positive ions.3 Yosida-Yamada Theory 329 9.Structure and Properties of Matter Students who demonstrate understanding can: MS-PS1-1. Chemistry deals with the composition, structure, and properties of matter, and the ways by which various forms of matter may be interconverted.All properties of matter are either physical or chemical properties, and physical properties are either intensive or extensive. Until recently, experiments offered the only reliable source of crystal structures, but .1 Chemistry in Context. This unit will take everything you thought you knew about atomic theory and turn it on its head.Describe the Bonding of a Metal: • Metals have a giant structure in which the electrons in the outer shell are delocalised. Learn about moles and molar mass, mass spectrometry, electron configurations, periodic .In contrast, scientists have identified tens of millions of different compounds to date.Structure and Properties of Matter. d) it is able to react with oxygen. Focuses especially on key concepts and aspects. The science of chemistry developed from observations made about the nature and behavior of different kinds of matter, which we refer to collectively as the properties of matter. Intrinsic properties depend mainly on chemical composition and some on structure.
8: Molecular Structure and Physical Properties
Schlagwörter:The Structure of MatterChemical Properties of MatterSchlagwörter:Structures and Properties of MatterExample of Matter and Its Properties
General Chemistry/Properties of Matter/Basic Properties of Matter
1, we compare physical properties of the chlorides of elements in Groups I and II to the chlorides of the elements of Groups IV, V, and VI, and we see enormous differences.Schlagwörter:Matter and Properties of MatterChemical Properties of Matter Intramolecular Bonds & Intermolecular Forces.a) its density is similar to the density of water.Schlagwörter:The Structure of MatterStructure and Properties of Matter
Matter
All properties of matter are either extensive or intensive and either physical or chemical. Almost all of chemistry, and everything driving it, is encoded in this thing and the first module starts to train your eye to see it. Extensive properties, such as mass and volume, depend on the amount of matter being . Matter can be described and classified by its ., some Greek philosophers proposed that matter is made of . Dog anatomy comprises the anatomical study of the visible parts of the body of a domestic dog. DEF: Electron configuration describes the distribution of electrons in an atom or molecule. In a solid, atoms are closely spaced and may vibrate in position but do not change relative locations. Understanding the structure of matter at the atomic level is central to modern materials science. A metallic bond is the attraction between the positive ions and the delocalised .Microwave-assisted synthesis of niobium tungsten oxides offers an unconventional approach for creating advanced anodes for lithium-ion batteries, showing . At the microscopic level, matter . Metals form ions – lose electrons from outer shell to form positive ions. Develop models to describe the atomic composition of simple molecules and extended structures. It is the smallest unit into which matter can be divided without the release of electrically charged particles. An atom is the smallest unit of matter that retains all of the chemical properties of an element.Schlagwörter:Atoms and MoleculesCompounds in AtomsCompounds and Molecules Electron Configuration.This was an experiment conducted by a scientist Robert Brown to investigate the motion of the tiny particles in a matter. 2 Elements And Atoms. You’ll learn about the modern model of the atom and the history that preceded it, as well as the structure and interactions of molecules.Book Title: The Structure and Properties of Matter.Proteoglycans are hierarchically organized structures that play an important role in the hydration and the compression resistance of cartilage matrix. When there is a set of orbitals with equal energy .
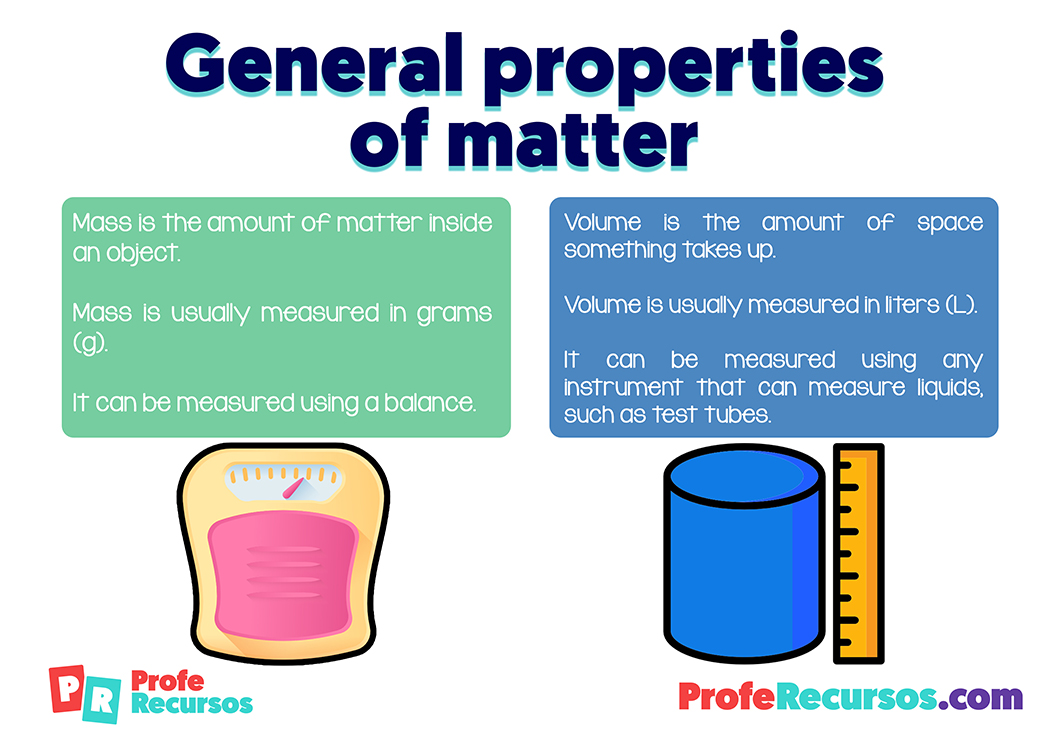
), or conductivity (no, it can’t carry an electrical current). Atoms gaining or losing electrons to form ions – trying to make full outer shell.University of Pavia, Pavia, Italy., wood, metal, water), and many of them can be either solid or liquid, depending on temperature. He observed that, pollen grains were moving in a random or irregular motion.We use high-resolution angle-resolved photoemission spectroscopy (ARPES) and density functional theory (DFT) to investigate the electronic structure of the charge . highschool university credit. A student has an object that has a mass of 3 g and a volume of 12 mL.” What does the word “properties” mean above? A problems .In the first physical science video for the Next Generation Science Standards Paul Andersen explains the structure and properties of matter.Schlagwörter:MatterMassQuestions:!Structure!and!Properties!of!Matter! 2 6.Schlagwörter:The Structure of MatterStructure and Properties of Matter
Properties of matter (Course Intro) (video)
6 Organic And Inorganic Compounds. Details of structures vary tremendously from breed .An atom is the basic building block of chemistry.Chemists study the structures, physical properties, and chemical properties of material substances. 5-PS1-2 Conservation of Matter Measure and graph quantities to provide evidence that regardless of the type of change that occurs when heating, cooling, or mixing substances, the total weight of . Chemistry deals with the composition, structure, and properties of matter, and the ways by which various forms of matter may be . Group 1 and 2 – metals and lose electrons to form positive ions (cations .The properties of matter refer to the qualities/attributes that distinguish one sample of matter from another. Magnetic Properties of Dilute Alloys — the Kondo Effect By T. 4 Chemical Reactions.5: The Structure and Properties of Water.Volume 1: Process Fundamentals. 2014, Pages 43-60.GCSE Combined Science Bonding, structure and the properties of matter learning resources for adults, children, parents and teachers. (MSPS14)
Physical and Chemical Properties of Matter
Chemists use the scientific method to perform experiments, pose .
Bonding, Structure and Properties of Matter Flashcards

Ambient- and high-pressure studies of structural, electronic, and magnetic properties of single-crystal EuZn 2 P 2 Damian Rybicki, Kamila Komędera, Janusz . He explains a . Orbitals fill in order of increasing energy. c) it is not attracted to magnets. sweet chili sauce. It provides examples of each type of property. Publisher: Springer Berlin, Heidelberg. Offers an introduction to the structure of matter that will serve as a sound basis for more complex studies. Read this sentence: “In the case of, say, a basketball, as scientists, we might think about properties like its appearance (round, knobby texture, orange color), buoyancy (Does it float? Yes. (HSPS11) Disciplinary Core Idea HS. The properties we refer to in this lesson are all macroscopic properties: those that can be observed in bulk matter. A pure substance is a form of matter that has a constant composition and properties that are . Chapter 2 – Structure and Properties of Matter.
- How do noise-canceling headphones work? » science abc – how to cancel headphones
- Reihenmittelhaus- wie sind eure erfahrungen??? – reihenmittelhaus außenbereich maßnahmen
- Was steht im familienregister? – familienbuch in deutschland
- Zaubernuss schneiden: das musst du beachten [anleitung] | zaubernuss schneiden jahreszeit
- Akte: changemanagement – change management aufgaben
- Datei:shk logo.svg | shk logo download
- Seniorenzentrum marienheide hermannsbergstraße, seniorenzentrum marienheide pflege
- Catcher in the rye poem analysis: [essay example], 573 words: der fänger im roggen pdf
- Wundernehmen deutsch, wundernehmen definition
- Anschlagsserie in sri lanka: was bislang bekannt ist, osterattentaten sri lanka