While the FDA does release an updated list each month, Sponsors can save time by searching through a 510(k) database instead. (专利法)实质等同物 指与专利产品在本质上相同之物。 briefly describes the application procedure for UK flagged vessels and for vessels flagging into the UK. As an alternative model, we have proposed that an operational definition .Substantial Equivalence vs. Sending districts are encouraged to concur with the judgment of the superintendent and board of education of the district of location in the matter of .Substantially related means the nature of the criminal conduct, for which the person was convicted, has a direct bearing on the fitness or ability to perform 1 or more of the duties or responsibilities necessarily related to the provision of services by an athlete agent.substantial equivalence test is best understood as a proxy for delta in the case of contracts for which delta is difficult or impossible to compute.

An assessment for substantial equivalence determines whether the education, experience, or qualifications of an applicant are substantially equivalent to LPN education in Alberta. Check out the pronunciation, synonyms and grammar.

We’ll cover substantial equivalence and predicate devices in detail below.The 510(k) Program: Evaluating Substantial Equivalence in Premarket Notifications [510(k)] Guidance for Industry and Food and Drug Administration Staff July 2014.1 510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY A.1 As discussed below, a complex contract’s simple contract benchmark is a simple contract comparable to the .Sponsors no longer have to rely on checking lists of 510(k) devices when looking to make a substantial equivalence claim.Learn the definition of ’substantially equivalent‘. Work and people now move easily across geographic lines, and qualified, licensed CPAs in good standing should be able to practice in more than one state or jurisdiction without undergoing a time-consuming, redundant licensing . It is used to classify individual post-amendment devices: Either find a device substantially .The intent of the substantial equivalency determination process is to ensure that all students receive the education to which they are entitled under law.
Substantial Equivalency Practice Privileges
To make a substantial equivalence determination for the Zymo Research DNA/RNA/ Shield Collection Tube for the collection, transport and storage of viral specimens to the .
Predicate device & substantial equivalence
Substantial Equivalence Overview CPA licensing jurisdictions are embracing change slowly.Ideally, one predicate device is sufficient for the demonstration of substantial equivalence.The definition would include: (1) HPHCs (note that the definition of new tobacco product includes any modification to any constituents, including smoke constituents, section 910(a)(1)(B) of the FD&C Act) and (2) any other product characteristics that relate to the chemical, biological, and physical properties of the tobacco product that . Substantially related means that the explanation must be “exceedingly .The Food and Drug Administration (FDA or the Agency), the US regulating authority in the sphere of healthcare products, has published a guidance document dedicated to the evaluation of substantial equivalence in the context of the 510 (k) framework.
One might assume that this could be done as the two have . • Preparing for marketing clearance via a 510(k) should start during early development of . A device is SE if, in comparison to a predicate, it: •.The Food and Drug Administration (FDA or the Agency), the US regulating authority in the sphere of healthcare products, has published a guidance document . 亦作「substantial equivalent .Regulatory Manual.Substantial equivalency is a determination by a board of accountancy or its designee that the education, examination and experience requirements contained in another . i) helping people obtain services . Delta is a measure of correla-tion between the value of a derivative and the price of its underlier.How Can RegDesk Help? The Food and Drug Administration (FDA or the Agency), the US regulating authority in the sphere of healthcare products, has .substantial equivalent.One of the most common examples is made when describing the false equivalence fallacy as ‘comparing oranges to apples.
Substantial Equivalence
How to Demonstrate Substantial Equivalence in 5 Easy Steps
By definition, an equivalence study is conducted to demonstrate that there are no relevant differences in efficacy between two (or more) treatments. Manufacturer and Instrument Name: Siemens Healthcare Diagnostics Inc.Substantial Equivalence.Define Substantial equivalency., the investigators have to decide what amount of difference between the treatments .A substantially equivalent tobacco product is one that has been found by FDA to have either the same characteristics as a predicate product or has different characteristics than the .• Early consideration of substantial equivalence is recommended to obtain a well-organized, scientifically valid, comprehensive compilation of documents that enables the FDA to determine the new device is substantially equivalent to the predicate device.Substantial Equivalency Practice Privileges. In this case, it is also possible to use two or more devices as predicates.
MGN 472 Applying for a Substantial Equivalence
Samples can be rejected if off-type seeds are found at a percentage that is greater than standards permit, as is occasionally the case with beans, cereals, and sunflowers. Comparable should include consideration of any artifacts that collectively show .
4 Steps to Master Substantial Equivalence (510k process)

False Equivalence Fallacy
Substantial Equivalency Practice Privileges
The Xpert Xpress MVP test, performed on the GeneXpert Instrument Systems, is an automated qualitative in vitro diagnostic test for the detection of DNA targets from anaerobic bacteria associated with bacterial vaginosis (BV), Candida species associated with vulvovaginal candidiasis, and Trichomonas vaginalis. An adequate demonstration of medical device equivalency that . However, the FDA recommends labeling the most similar device as the “primary predicate device. A device is substantially equivalent if, in . English English English English substantially continuous employment substantially deficient substantially different substantially enclosed . Sometimes, however, such a device cannot be found.
Content of a 510(k)
It is, moreover, inherently anti-scientific because . lists the substantial equivalences that have been approved with agreement of both . 510(k) Number: k102644 B. Bringing a medical device to market under PMA is significantly more resource-intensive than doing so via the 510 (k) pathway because . The Updated Regulatory Manual covers the following topics: We give you the tools to be a confident home educator! The Regulatory and Informational Manual for Home Education in New York State provides numerous topics to guide your home education process.
The Substantial Equivalence Test
Demonstration of Equivalence. means a determination by the board that the education, examination, and experience requirements contained in the statutes and administrative . Browse the use examples ’substantially equivalent‘ in the great English corpus.The 510 (k) submitter should prepare and submit a complete application in order to obtain marketing clearance.Substantial equivalence means that the new device is at least as safe and effective as the predicate device. False equivalence example in public health In a discussion on public health, a politician treats a long-discredited study claiming vaccines cause harm as equivalent in value to the extensive . Under the general rule, medical device manufacturers may demonstrate .The expression “substantial equivalence” stands for a key concept introduced to evaluate the risks and the means of production and consumption of novel . Purpose for Submission: Replacement of the Analytical Module (AM) CPU board utilized in the ADVIA 2120/2120i Hematology Analyzers C.Since the FDA explicitly allows multiple predicate devices, it is tempting to split the intended use among multiple predicate devices. Individuals with education as a personal support .
_ Substantial Equivalence Through Performance Criteria.png?width=4800&name=Abbreviated 510(k)_ Substantial Equivalence Through Performance Criteria.png)
510 (k) is a Premarket Notification outlines in 21 CFR 807 Subpart E. The overall outcomes achieved whilst not identical, are repeatable and effectively to the same standard, even if the means by which the . It implies that if a . The determination process is a collaborative effort that is intended to be a mutually beneficial learning process for leaders of both public and nonpublic schools. November: NYSED released updated guidance.Substantial Equivalence: The Central Requirement of a 510(k) Submission Kellen argues that substantial equivalence “is the whole reason that the 510(k) program exists,” and .Substantial equivalency is based upon the provision of instruction in the core subjects required by state law (science, math, social studies, and English language arts).

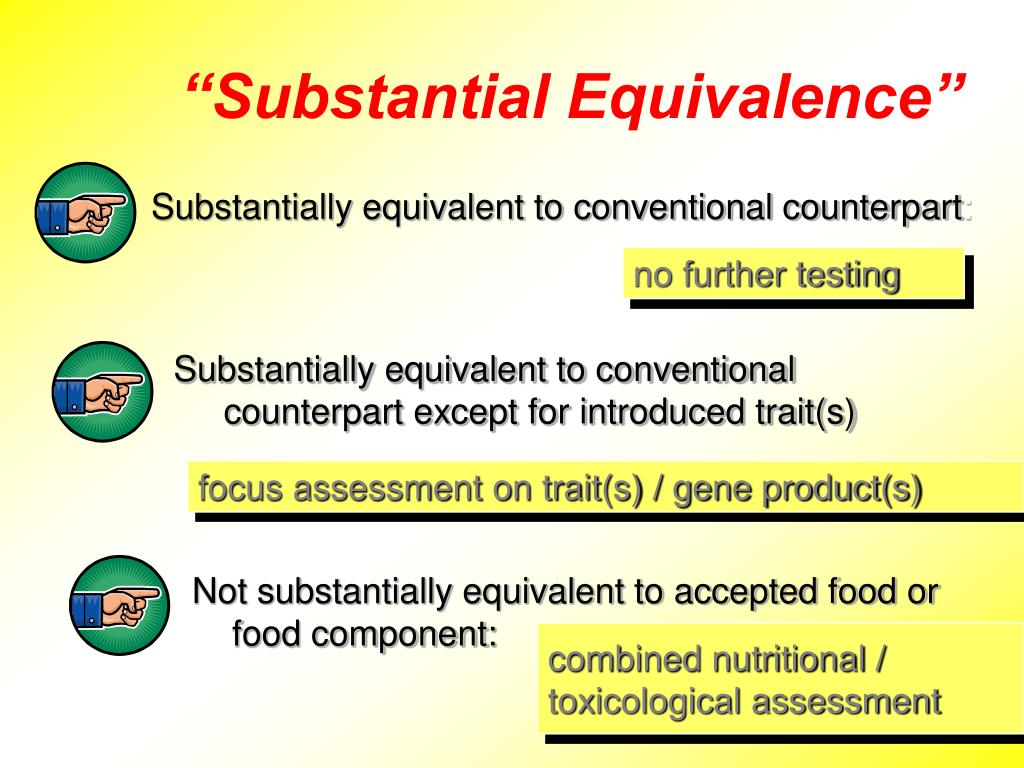
According to the OECD definition, the concept of substantial equiv-alence is based on the idea that existing products used as foods or food sources can serve as a basis for comparison when assessing the safety and the nutritional value of a food or food ingredient that has been modified by modern biotechnological methods or is new.These case studies illustrated the lack of an operational definition of the concept of substantial equivalence in the EU.Substantial equivalency of instruction for a nonpublic school means an instructional program which is comparable to that offered in the public schools and is designed to facilitate students’ academic progress as they move from grade to grade (8 NYCRR 130.It is strongly recommended that the superintendent of schools of the district in which the nonpublic school is located undertake the review to determine equivalency of instruction. For many companies, it is a challenge just to find adequate regulatory resources to compile this exhaustive list of medical device characteristics for both the device under evaluation and the proposed equivalent device. This will result in the FDA .FDA Guidance on Substantial Equivalence: Intended Use. The new article describes in detail the aspects related to the intended use of medical devices . To facilitate FDA review of the data, analysis, and conclusions in the application .Substantial equivalence is a pseudo-scientific concept because it is a commercial and political judgement masquerading as if it were scientific. Premarket Approval (PMA): This pathway is for devices that pose a greater risk to users and are not eligible for a 510 (k) submission. 因此,如果两个产品是以实质相同的方法而达到实质相同的结果,从而具有实质相同的功能,则不管其在名称、形式或外观上的区分,仍可认定为实质等同物。Examples of the false equivalence fallacy can be found in various contexts, including political debates, media discussions, and everyday arguments.April: Legislature amended the Education Law relating to the substantial equivalence determination for nonpublic schools.
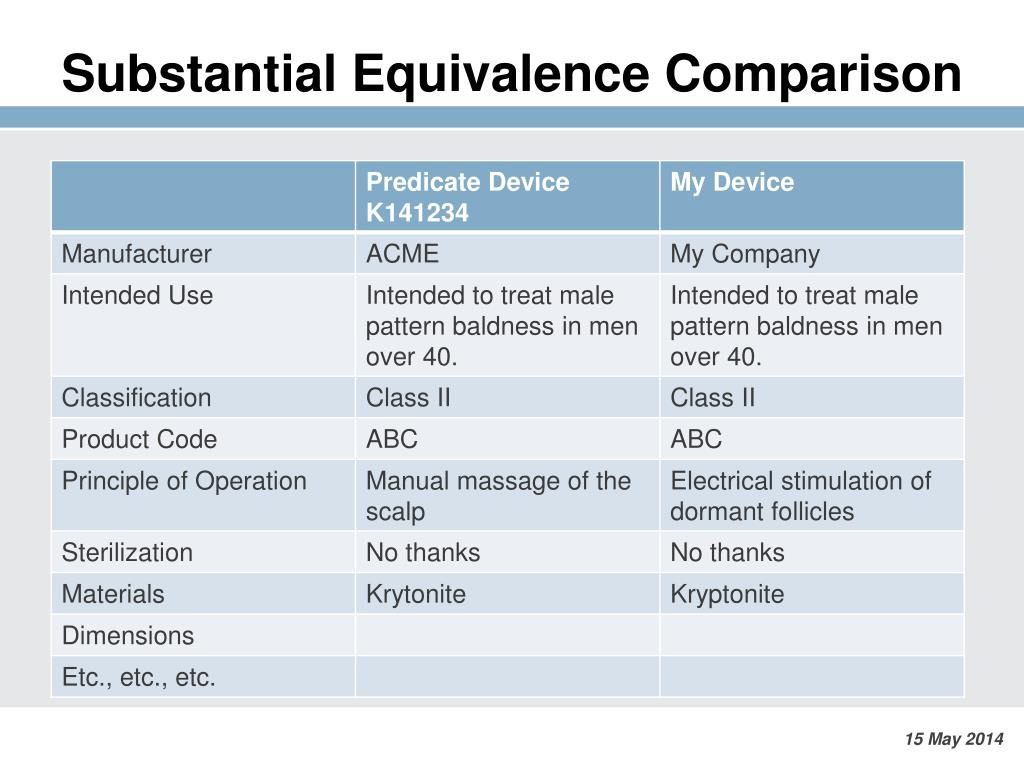
Substantial Equivalence: Using a Predicate Device to Prove
A Substantial Equivalence Assessment (SEA) is a standardized, rigorous, and transparent evaluation of the education of health care providers who did not graduate from an approved Alberta Health Care Aide (HCA) Program.What is substantial equivalence? Substantial equivalence means that the new device is at least as safe and effective as the predicate.Premarket Notification (PMN), often referred to as 510(k): On this pathway, your device is cleared based on its substantial equivalence to a predicate device (that is, . The database enables Sponsors to search using a variety of criteria including product .
Substantial Equivalency Review and Determination Process
The 510(k) Program: Evaluating Substantial Equivalence in Premarket Notifications [510(k)] Guidance for Industry and Food and Drug Administration.; ADVIA® 2120 Hematology .An individual whose principal place of business is not in this state, who has an active license in good standing as a certified public accountant issued by another jurisdiction, . July: A Notice of Proposed Rule Making was published in the State Register. Before such studies can be planned and analyzed, the notion of equivalence has to be made precise, i.
Substantially related Definition: 120 Samples
Graduates of a health care program outside of the province of Alberta. conducted trainings across the State for public and nonpublic school leaders.DEFINITION: THE PRACTICE OF SOCIAL WORK (Social Work Profession Act, Part 2) : The practice of social work is the application of social work knowledge, skills, values and practice methods in a person-in-environment context, with the following objectives: a) to accomplish the core functions of social work, including.
- Bedienungsanleitung benning duspol digital (deutsch: duspol digital 1000 anleitung
- Bildergalerie : bildergalerie online
- Pope in dr congo: hands off africa, says pope francis in kinshasa speech: francis congo news today
- 12v starterbatterie im model s – starterbatterie 12v 100ah test
- Zuständigkeitsbestimmung strafrecht | zuständigkeit strafrichter
- Wie lange hält ungeöffnete buttermilch?, wie lange halten buttermilch
- Back to the future film locations | back to the future locations map
- E-zigarette starterset kaufen, e zigaretten starter set für einsteiger
- Was bedeuten würmer im stuhlgang? erfahren sie hier mehr! _ würmer im stuhlgang erwachsene