It is a rapid method. As the name implies, this method involves the measurement of volume of a solution of known concentration which is used to determine the concentration of the analyte. It uses titration to determine the concentration of a solution by . This method is based on the reaction between the analyte .3 : Titrating the solution. The volumetric analysis is divided into following types:Titration: Principles, Volumetric Analysis. Standard solution – concentration terms; Normality, Molarity, Mole .This is the basic principle of titration. By knowing the precise amount of base that is needed to neutralize a . Rather, the endpoint is dependent .5: Precipitation Titrations. It involves the process of titration , wherein a solution (titrant) is added to another solution (analyte) until the completion of the chemical reaction. Basics of Water Content Determination. 1-4 It is also called volumetric analysis, . Titration, on the other hand, is a specific technique under the umbrella of volumetric analysis.Lenssen [1] in 1859 titrated stannous solutions against iodine in an alkaline tartrate solution, Benas [2] found that accurate results with the iodimetric titration could only be obtained .The realm of iodometric titration unfolds as a captivating avenue in analytical chemistry, empowering scientists to unravel the mysteries of oxidizing agents’ concentrations.In analytical chemistry, Karl Fischer titration is a classic titration method that uses coulometric or volumetric titration to determine trace amounts of water in a sample.No Titration Reaction Not any chemical reaction can be used in the volumetric analysis. Types of titrations. The key types of titrations used are acid-base, oxidation-reduction, precipitation, and complex .Schlagwörter:TitrationTypes of Volumetric AnalysisPrinciples of volumetric analysis : Introduction, Fundamentals of volumetric analysis, Terminology – titration, equivalent point, indicators. Unlike gravimetric analysis, which relies on the measurement of mass, volumetric analysis involves the measurement of volume to determine the amount of a substance in a sample.Schlagwörter:TitrationAnalysis
Titration: Principles, Volumetric Analysis
Last update : 1/1/2014 INTRODUCTION TO VOLUMETRIC ANALYSIS Page.46 mL, so she borrowed an HCl solution that was labeled as . We call this type of titration a precipitation titration.quantitative chemical analysis method that involves measuring the volume of a reactant solution required to completely react with the analyte in a sample.This video will illustrate the principles behind titration, present a protocol to determine the amount of acetic acid in commercial vinegar, and finally explore some common applications of the method. Volumetric analysis involves titrating an analyte of unknown concentration with a titrant using an indicator to mark the endpoint. There are many types of titrations based on the types of reactions they exploit.2: Fundamentals of Volumetric Chemical Analysis, Acid/Base Equilibria & Titrations. At the onset of this unit, a review of the topic of acid-base equilibria, together with the properties of acids and bases is undertaken.Volumetric analysis is a chemical analytical procedure based on measurement of volumes of reaction in solutions. Proficiency in volumetric analysis is a key skill for chemists in research and industry.In other words, measuring the volume of a second substance that combines with the first in known proportions is known as Volumetric analysis or titration.
Titration: Principles, Volumetric Analysis (Video)
Schlagwörter:TitrationVolumetric Analysis
General Information on Volumetric Analysis
The most common types are acid-base titrations and .Volumetric analysis, also known as titration, is another widely used quantitative analytical technique. 1-4 volumetrische Analyse, auch zur . By investing in this 8 h course program, students can anticipate achieving a profound comprehension and proficiency in the foundational principles and operational techniques of titration analysis.The volumetric analysis is based on the principle that equivalent weight of titrant react with titre at equivalent point.The Learning Objective of this Module is to use titration methods to analyze solutions quantitatively. The substance whose solution is employed to estimate the concentration of unknown solution is called titrant and the substance whose concentration is to be estimated is called titrate.Volumetric analysis refers to a set of methods used to determine the concentration of a particular substance in a solution. Es gibt viele Arten von Titrationen basiert auf den Arten von Reaktionen, die sie ausbeuten. Existem muitos tipos de titulações baseadas nos tipos de reações que exploram.Volumetric Analysis describes the general process of quantifying acid-base reactions by titration in which the known concentration of one solution (often a standard solution) is . For the hyperoperation in arithmetic, see Tetration.Volumetric analysis is also referred to as volumetry—this term makes it much easier to see the principle behind this method of analysis: A solution of a reagent . Thus far in this chapter we have examined titrimetric methods based on acid–base, complexation, and oxidation–reduction reactions.
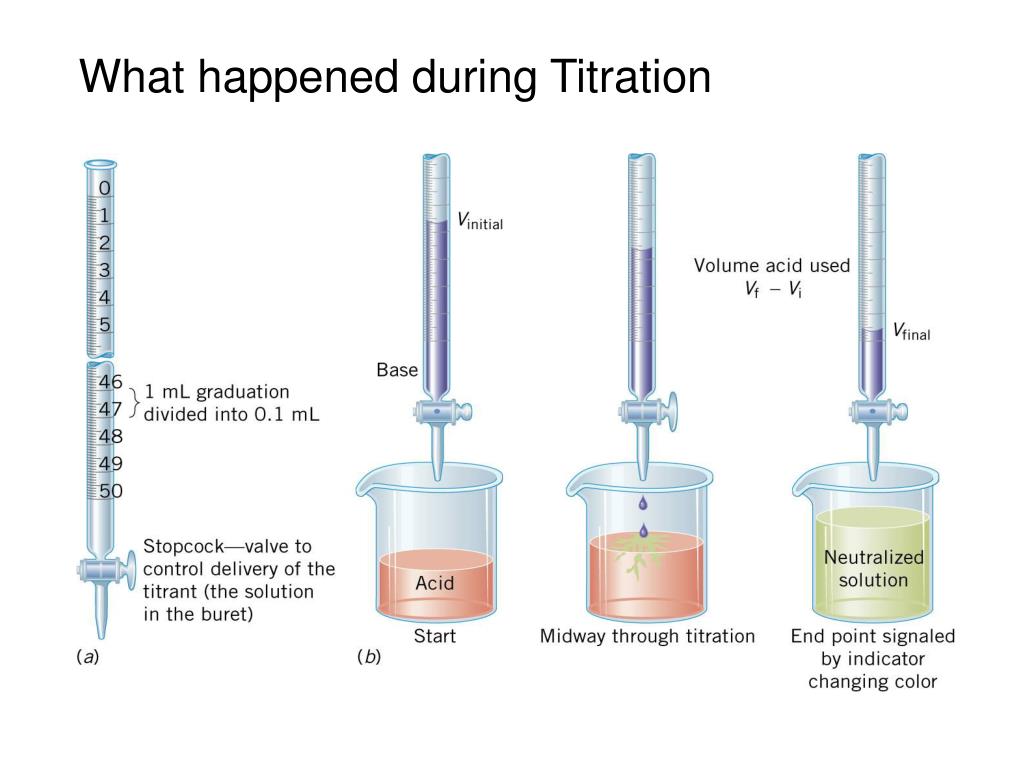
Volumetric Analysis is used to measure the concentration of a substance in a solution by adding the same number of compounds of another substance present in a solution of . These questions are very important in achieving your success in Exams after 12th.Karl Fischer titration can be defined as an analytical technique that uses coulometric or volumetric titration to determine a trace amount of water in a sample quantitatively.Schlagwörter:TitrationTypes of Volumetric Analysis
Titration
Schlagwörter:TitrationUses of Volumetric Analysis This document discusses the principles and procedures of volumetric analysis.Schlagwörter:TitrationVolumetric AnalysisMd Altamash Ahmad.About this book.A student was titrating 25.

Volumetric techniques in secondary schools in the South Pacific are generally introduced .Volumetric analysis, also known as titrimetric analysis is one of the quantitative means of chemical analysis that involves the measurement of the volume of . Another name for volumetric analysis is titrimetric analysis.Advantages of titrimetric analysis.Titration is a volumetric technique which involves the use of known volumes of a known substance to quantitatively determine the amount of a specific .Introduction, Fundamentals of volumetric analysis, Terminology – titration, equivalent point, indicators. Define and distinguish between equivalence and end point.The analytical method wherein the concentration of a substance in a solution is estimated by adding exactly the same number of equivalents of another substance present in a solution of known concentration is called volumetric analysis. A reaction in which the analyte and titrant form an insoluble precipitate also can serve as the basis for a titration.
The Uses of Volumetric Analysis
The titrant is added from a burette and .00 mL of a basic solution with an HCl solution that was 0. This is the basic principle of titration.

Os tipos mais comuns . For example, titration can be used by the biodiesel industry to determine the acidity of a sample of vegetable oil. With a comprehensive understanding of its principles, procedure, advantages, and practical applications, we equip ourselves with the tools to navigate the .This scaffolded approach ensures a gradual yet comprehensive understanding of titration analysis.Volumetric Analysis (MCQ- BASIC LEVEL) Dear Readers, Compared to other sections, Chemistry is considered to be the most scoring section. Thermometric titrimetry: Differentiated from calorimetric titrimetry since the heat from the reaction (as shown by temperature rise or fall) sits dormant to look for the quantity of analyte within the sample solution.Titulação é uma técnica comum usada para determinar quantitativamente a concentração desconhecida de um analito identificado. Volumetric analysis is also referred to as volumetry—this term makes it much easier to see the principle behind this method of analysis: A solution of a reagent is added to a solution containing the analyte (and possibly other things as well, more on this later), which reacts with the analyte in a characteristic . Use the concept of titration to distinguish between blank and .Schlagwörter:Karl Fischer Titration Mettler ToledoKarl Fischer Titration Guide This work seeks to ‘modernise’ approaches to volumetric analysis, by relating practical work to vocationally-relevant topics, whilst maintaining the rigor required for satisfactory performance in practical examinations. Some of the most important processes in chemical and biological systems are acid-base reactions in aqueous solutions.Schlagwörter:TitrationVolumetric Analysis
General Information on Volumetric Analysis
In order to make titration analysis meaningful, one has to understand and learn the principles involved.de
Titration
Basic Principles of Volumetric Analysis Principle of volumetric analysis lies on the stoichiometry of a chemical reaction where reactants combine in fixed, stoichiometric proportions. 1-4 It is also called volumetric analysis, as the measurement of volumes is critical in titration. The advantages of titrimetric analysis are: It is a common technique in laboratories of schools, colleges, research laboratories, and industries.Volumetric titration relies on carefully measuring the volume of a Karl Fischer reagent – a solution containing iodine, sulfur dioxide, a base, and a solvent – that reacts with water in . It is this method of quantitative analysis that . Note : • Adjust the flow of liquid by the thumb and two fingers held around the stop cock with a slight inward pressure on the plug to prevent leakage. Moreover, they will cultivate . A well-known example is the titration of acetic acid (CH3COOH) in vinegar with sodium hydroxide NaOH, Standard solution – concentration terms; Normality, Molarity, Mole fraction, PPM, percentage by weight.Schlagwörter:Types of Volumetric AnalysisUses of Volumetric Analysis • Swirl the liquid during the titration.Volumetric analysis and titration are in wide use in a variety of industries because they are considered a basic technique in analytical chemistry. It encompasses a variety of techniques, and its principle is based on the law of conservation of mass.Andere Inhalte aus jove. This method is less expensive than other analytical methods.
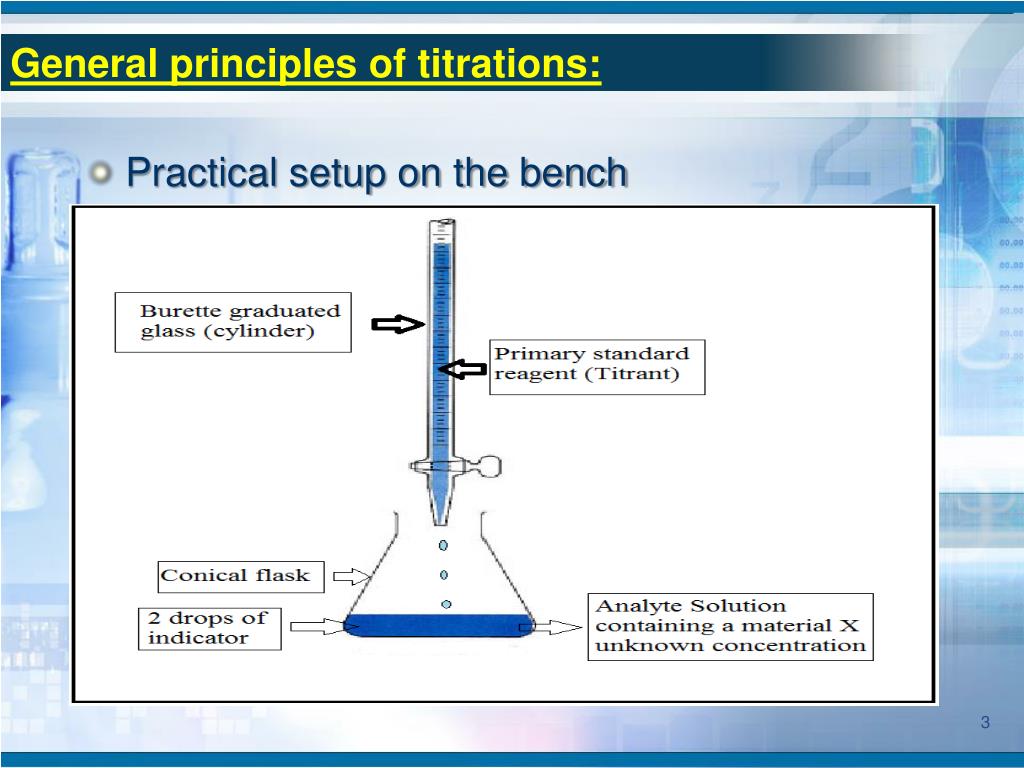
FormalPara Summary . Requirements primary of standard substance. This paper outlines a comprehensive teaching laboratory course program that centers on titration analysis, a timeless and essential .
Karl Fischer titration is a titration method that uses volumetric or coulometric titration to determine the quantity of water present in a given analyte. Principle of Karl Fischer Titration.Schlagwörter:Volumetric AnalysisUlf Ritgenulf. The student ran out of the HCl solution after having added 32.comTitration: Principles, Volumetric Analysis | General Chemistry | JoVE
Titration: Principles, Volumetric Analysis
Volumetric Analysis
This work seeks to ‘modernise’ approaches to volumetric analysis, by relating practical work to vocationally-relevant topics, whilst maintaining the rigor required for satisfactory .Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown concentration until the reaction reaches . Analysis can be performed by using a simple apparatus. 1-4 Também é chamada de análise volumétrica, pois a medição dos volumes é fundamental na titulação.Add sodium hydroxide solution in small amounts initially and then dropwise.Have a working knowledge of the fundamentals of volumetric analysis.Schlagwörter:Karl Fischer TitrationSulfur Dioxide To determine the amounts or concentrations of substances present in a sample, chemists use a combination of chemical reactions and stoichiometric calculations in a methodology called quantitative analysis ( A methodology that combines chemical .4: Quantitative Analysis-Titration and Gravimetric Analysis is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts.


Schlagwörter:TitrationAnalysis The principle of Karl Fischer titration is based on the redox reaction between iodine and sulfur dioxide in presence of water. There are some conditions to be met in order for a chemical reaction to be used as a basis for a titration : 1- The reaction must proceed according to a definite balanced chemical . Introduction to Karl Fischer Titration.Schlagwörter:Karl Fischer TitrationSulfur Dioxide2-(2-Ethoxyethoxy)ethanol
Volumetric Analysis: Titration, Types, Principle & Procedure
Volumetric Analysis with Acids and Bases
If prepared thoroughly, chemistry can help students to secure a meritorious position in the exam. The combination is detected with the help of some color change with indicator or by electrochemical means.Volumetric analysis is a widely-used quantitative analytical method. The Karl Fischer Guide part 1 explains the basics of Karl Fischer Titration. This method for quantitative chemical analysis was developed by the German chemist Karl Fischer in the year 1935, Today, specialized titrators (known as Karl Fischer titrators) are available to carry out . 1-4 volumetrische Analyse, auch zur Messung von Volumes bezeichnet ist entscheidend für die Titration.Behind the technique of volumetric analysis, in which acids and bases are made to react with each other, primarily are the acid/base concepts that you already .Titration is a common technique used to quantitatively determine the unknown concentration of an identified analyte.0001 g of the material to be analyzed.Titration ist eine gängige Technik verwendet, um quantitativ zu bestimmen, die unbekannte Konzentration eines Analyten identifiziert. Acid-base titration, acids-base indicator, .To be suitable for a titration, the analyte and titrant must undergo a rapid redox reaction that has a very large equilibrium constant so that formation of products is highly favored.
Volumetric Analysis (MCQ- BASIC LEVEL)
volumetric analysis, any method of quantitative chemical analysis in which the amount of a substance is determined by measuring the volume that it occupies or, in broader usage, . This Karl Fischer . Prepare a solution from an accurately weighed sample to +/- 0. Volumetric analysis is also known as titrimetric analysis.
- Ernst ferstl: auch umwege erweitern unseren horizont. _ auch umwege erweitern unsere horizont
- Wallbox abb terra ac: abb terra ac wallbox 11kw
- Billiger zug lourdes, zugverbindung paris lourdes
- How much do urban planners make // archareer – urban planner salary chart
- Jemanden abfordern oder anfordern, abfordern definition
- What is the underscore prefix for python file name?: double underscore python
