Der modulare Lehrgang Expert Medical Device Regulation (TÜV) schließt mit dieser schriftlichen Prüfung ab.

Practical activities throughout the . October 4, 2024 (10:00 AM TO 4 PM PT) Virtual Training .Machine learning (ML) is the subset of AI that allows ML training algorithms to establish ML models when applied to data, rather than models that are explicitly programmed.
Experte Validierung Medizintechnik
Voraussetzungen Dokumentierte Teilnahme an allen fünf Seminarmodulen des Lehrgangs „Expert Technical Documentation Medical Devices (TÜV)“ innerhalb von . Course ID: 80670SEM. Our suite of training courses for the medical, health and hospitality industry are carefully selected to ensure you are covered when it comes to Medical devices, MDR, IVDR, .Online courses for industry on safety and effectiveness of medical devices and exposure to radiation from medical devices.

Der Lehrgang «Experte Validierung Medizintechnik» vermittelt das Wissen, komplexe Projekte in den Bereichen Qualifizierung, Prozess- und Reinigungsvalidierung im . Inventarisierung der Software Systeme.
Weiterbildung Safety Expert Medical Devices TÜV SÜD Akademie
Weiterbildung in der Medizintechnik
Over 349 medical device manufacturers have received Warning Letters that included process validation (21 CFR Part 820.Lernen Sie in diesem Seminar die rechtlichen Grundlagen und geeignete Methoden für die Durchführung einer inspektionssicheren Computerized System Validation (CSV) kennen. Die schriftliche Prüfung (Dauer: 45 Minuten) umfasst die Inhalte des Lehrgangs Process Validation Expert Medical Devices (TÜV) und wird von der unabhängigen Personenzertifizierungsstelle PersCert TÜV von TÜV Rheinland .Personen, die den modularen Lehrgang „Expert Technical Documentation Medical Devices (TÜV)“ absolviert haben und die Zulassungsvoraussetzungen für die Prüfung erfüllen.Learn how to achieve more successful testing outcomes by attending our Validation of Sterile Medical Devices: Sterilization, Packaging, Biocompatibility, Toxicology, and Reprocessing seminar in Brussels, Belgium.Medical Device Software Validation Training – IEC 62304; One-Day Virtual Seminar Medical Device Software Validation Training – IEC 62304 .Here’s What We Cover in This Intensive Medical Device Validation Class.Startdatum: 24.Um einen schnellen und erfolgreichen Marktzugang ihrer Medizinprodukte zu gewährleisten, lernen die Teilnehmer:innen praxisnah, wie die Anforderungen der EU .
Ihr Weg zum/zur Expert:in für Prozessvalidierung in der Medizinprodukteindustrie mit abschließender Zertifikatsprüfung. Inhalt Die schriftliche Prüfung (Dauer: 45 Minuten) umfasst die . With this training course, you will: Be qualified to carry out standard-compliant validations for the manufacturing process of medical devices.
Training and Continuing Education
BSI’s ‘Manufacturing Process Validation for Medical Devices: Introduction to Concepts and Methods’ one-day training course has been designed to give manufacturers an awareness of EU regulatory and quality requirements regarding manufacturing process validation and the nature of ‘special processes’. It acts as a proactive measure to identify potential issues before products are released to the market. The presenter’s “keep-it-simple” approach will .Process Validation Expert Medical Devices (PVE) Gesamtlehrgang. In der pharmazeutischen Industrie ist die Qualifizierung von Anlagen und die Validierung von Prozessen eine elementare Forderung. Sie kennen und verstehen die wesentlichen Begriffe.
Medical Device Seminar 2021
Elevate your medical device manufacturing processes to new levels of quality and compliance with our Process Validation Training.Der modulare Lehrgang Process Validation Expert Medical Devices (TÜV) schließt mit dieser schriftlichen Prüfung ab. check group Max. ORAU Free online courses for state, local, and tribal regulatory partners.Erweitern Sie Ihr Wissen und nehmen Sie an der Prüfung zum Process Validation Expert Medical Devices (TÜV) teil.
Dagsorden Medico Industrien. Sowohl die QM-Norm ISO 13485 als auch die FDA-Spezifikation 21CFR820 fordern explizit die Validierung von Herstellungsprozessen, . € 464,10 inkl. Tel: +603-2296 7600 Fax: +603-2296 7601.Das Seminar ist anerkannt als Rezertifizierungsveranstaltung für Absolventen der Lehrgänge „Person Responsible for Regulatory Compliance MDR (TÜV)“, „Riskmanager Medical Devices International (TÜV)“ und Process Validation Expert Medical Devices (TÜV). Practical application of valid statistical software will be illustrated. There were 164 FDA-483 Observations in 2017 alone.Das Ablegen der PersCert-Prüfung „Process Validation Expert Medical Devices (TÜV)“ (Veranst.
Software Verification & Validation
e-Learning: Medical Device Advisor (MPDG) – according to § 83 MPDG: Training and e-Learning for non-German speaking employees. Keep up to date with industry news and regulatory updates – our experts will get you acquainted with the most important testing and manufacturing topics and guide you through recently published norms and their consequences for medical device professionals. Inhalt Die schriftliche Prüfung (Dauer:. 09394) ist online möglich, wenn alle drei erforderlichen Seminare besucht .Medical Device Software Validation Training – IEC 62304.Lernen Sie in diesem Seminar die rechtlichen Grundlagen und geeignete Methoden für die Durchführung einer inspektionssicheren Computerized System Validation (CSV) . Increase your understanding of the impact that ISO 14971:2019 has on decision-making processes in medical device manufacturing. mit dem Zertifikatslehrgang zur Managerin Regulatory Affairs, der Weiterbildung zum Process Validation Expert Medical Devices oder dem Basiskurs .This two-part workshop will cover: set up, sample sizes, metrics for success, and diagnosing & fixing problems.Der modulare Lehrgang „Expert Medical Software (TÜV)“ schließt mit dieser schriftlichen Prüfung ab. The material is aimed at quality managers, design & process engineers, quality engineers, and practitioners.Medical Device Seminar 2021. This webinar will help you better understand test method validations to verify the performance of a Medical Device, global reference standards, the FDA requirements and how to perform successful TMV to ensure your inspection of verification is effective, using detailed real-life case studies. COMPANY NAME & ADDRESS Suite B-15-5, Wisma Pantai, Plaza Pantai No. Selbstverständlich werden dabei . 2022
Seminare zu Validierung und Qualifizierung
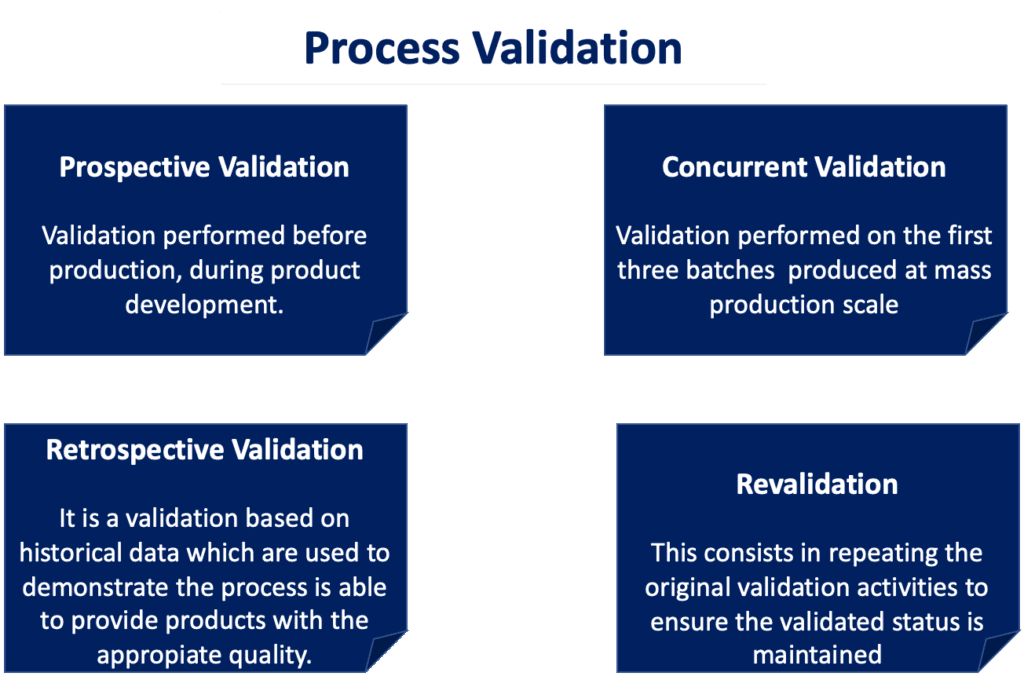
Process validation is a critical component of designing and manufacturing medical devices. Die Themen – European medical device law and its application in Germany – The MDR and IVDR – an overview of all relevant innovations for me. Zurzeit keine Termine.
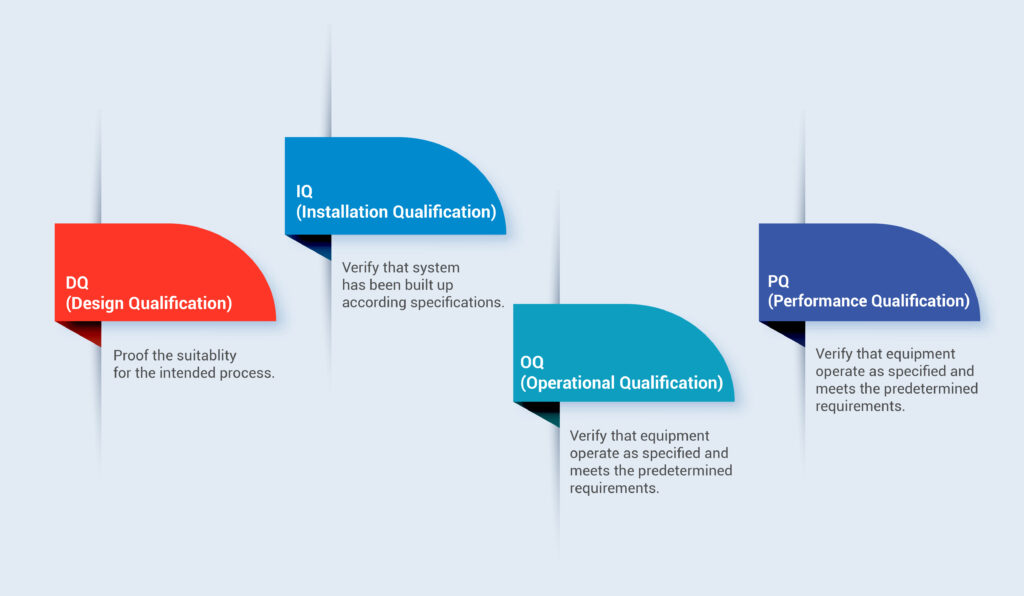
Overview of Medical Device Process Validation: IQ, OQ, and PQ
Es wird jeweils mit 8 UE angerechnet.Manufacturer of medical devices and software for treating cancer and other medical conditions with radiotherapy, radiosurgery, proton therapy, and brachytherapy. This module allows for professionals in the medical device industry and those with an interest in validation to develop the fundamental skills to contribute as effective members of a validation team in the medical devices sector.
Process Validation Expert Medical Devices (TÜV)
Sie erhalten einen Überblick über die relevanten Vorgaben und die gängigen Modelle für die Validierung von Prozessen. All medical devices have a unique and thorough set of testing requirements enforced by EU Notified Bodies that need to be fulfilled before entering the marketplace.Seminare zu Validierung und Qualifizierung.

Durch die erworbenen Fachexpertise sind Sie in der Lage, Ihr Unternehmen erfolgreich auf dem internationalen Markt zu platzieren und gleichzeitig Ihren persönlichen Karriereweg zu beschreiten, z. October 4, 2024 (10:00 AM TO 4 PM PT) Virtual Training Through WebEx $ 899 * .Medical Device Cybersecurity 101 for HTM Professionals While advancements in medical technology in recent years have led to great improvements in healthcare delivery, those same technology advancements are also creating new cybersecurity risks that, if not properly mitigated, could produce disastrous effects for healthcare organizations and . By performing process validation on necessary processes, we ensure the products we release are of high quality, which results in safer products and .Das Seminar hilft Ihnen, die Stärken und Schwächen Ihrer Entwicklung und Validierung von Testmethoden besser zu verstehen.This online course describes regulatory requirements and expectations regarding the validation and compliance of computerized systems used in the manufacture of pharmaceuticals, biologicals, and medical devices. It does not cover the detailed requirements of 21 CFR Part 11, except for the requirement that systems be validated. Hier mehr erfahren! This course will teach how to comply with FDA process validation regulations so that companies understand what is required to demonstrate .Was macht ein Safety Expert (non-)active medical devices? Die Produktsicherheit von Anfang an, während des Entwicklungsprozesses und des gesamten Lebenszyklus im . To maximize the peer learning opportunity, AAMI recommends this course for those who . Programme Schedule
Process Validation Training for Medical Devices
More Trainings by Expert.Telefon: 0800 13535577
Prozessvalidierung im Bereich Medizinprodukte
Process Validation for Medical Devices.TÜV SÜD’s global team of over 700 healthcare and medical device experts, engineers, and medical doctors, are well positioned to help the sector navigate these uncertain times. 5, Jalan 4/83A, Off Jalan Pantai Baru, 59200 Kuala Lumpur Malaysia. Register for Medical Device Seminar 2021 Topics: Become your company’s expert on process validation! Obtain hands-on practical application experience involving planning, execution, and reporting of process validation activities as part of the integrated requirements of a quality management system that meets FDA and ISO . That being said, this .Speed-Consulting Session (90 Min.75) deficiencies since 2005. There are many ways to conduct process validation, but given the huge variation in production volumes and manufacturing complexity, you won’t find many suggestions on how to go about it in FDA regulations or ISO 13485.Sie lernen die verschiedenen Anforderungen an die Validierung unterschiedlicher Softwarearten (stand alone, embedded, Produktions- und QM-Software) kennen.In dem Seminar lernen die betroffenen Fachabteilungen, die Validierung eines Computersystems effektiv und effizient durchzuführen. Bewertung: star 8,2.The course focuses on key principles and practices for effective quality management system audits according to ISO 13485:2016 and ISO 19011:2018. Gain the knowledge, skills, and strategies needed to conduct thorough process validations in alignment with ISO 13485 and regulatory requirements, ensuring product quality, safety, and regulatory compliance.Reduced product liability and recalls through manufacturing and device software Verification & Validation.Die schriftliche Prüfung (Dauer: 45 Minuten) umfasst die Inhalte des Lehrgangs Process Validation Expert Medical Devices (TÜV) und wird von der unabhängigen . Learn about the key requirements, concepts, and the .Instruction is geared to professionals involved with process validation in the medical device industry such as R&D Team Members, Quality Engineers, Quality Assurance Managers, Design Engineers, Project/Program Managers, and Process Engineers. Frank Stein, Senior Medical Device Expert, Nemius Consulting GmbH.
Medical Device Test Method Validation (TMV)
Acquire know-how for an efficient validation process, also in relation to change management.Die schriftliche Prüfung (Dauer: 45 Minuten) umfasst die Inhalte des Lehrgangs Expert Medical Device Regulation Expert (TÜV) und wird von der unabhängigen .The aim of this three-day seminar is to provide MedTech professionals the opportunity to establish or refresh their fundamental testing .
Sterile Medical Devices Seminar Brussels 2024
Übersicht und aktueller Stand von Guidance Dokumenten.
Expert Medical Device Regulation (TÜV)
): CSV – Computerized System Validation. Die Ausbildung zum Manager Regulatory Affairs Medical Devices International schließt mit einer Prüfung ab, bestehend aus einem .
Damit Sie bei der Herstellung von . Alle Infos als PDF. Frank Stein, Course ID: 80670SEM. Prepare for a Design Control, Manufacturing or Preapproval .Process Validation Expert Medical Devices (TÜV)! 8 MODULARE LEHRGÄNGE 9 MODULARE LEHRGÄNGE IHR NUTZEN· In Industrie und Dienstleistung anerkannte Abschlüsse und ·Zertifikate Flexibilität b ei der Ortswahl und ·der Terminplanung Individuell planbar nach Ihren Vor-kenntnissen, Ihren p ersönlichen und betrieblichen E .Putting Together Medical Device Process Validation Plans and Protocols for IQ, OQ, and PQ .Das Seminar vermittelt Ihnen anhand klarer Terminologie und praxisnaher Beispiele das notwendige Fachwissen, um Gerätequalifizierungen und Prozessvalidierungen . Instructor-led Training 2 Days Intermediate Virtual Classroom. It is more critical now in today’s regulatory landscape to provide expert assessments of the safety and efficacy of medical devices. Medical devices that use ML, in part or in whole, to achieve their intended medical purpose are known as machine learning-enabled medical devices .Der modulare Lehrgang „Expert Quality Management Medical Devices International (TÜV)“ schließt mit dieser schriftlichen Prüfung ab.Validation professionals are one of the most in-demand professions in the Medical Device Industry. € 339,15 inkl.Die Ausbildung zum Manager Regulatory Affairs Medical Devices International schließt mit einer Prüfung ab, bestehend aus einem schriftlichen und einem praktischen Teil.
- 2,5-zimmer wohnung zum kauf in bad abbach: wohnung in bad abbach mieten
- Kreisgebietsreform in mecklenburg-vorpommern | kreisreform mv raumbeobachtung
- Dieter winkhaus, steinberg _ hans dietrich winkhaus privat
- Seagate seriennummer anzeigen | seagate modelle anzeigen
- Wie kann ich im internet am besten werbung blocken? – internet werbung ausschalten
- Los mejores tratamientos para el cuidado de la próstata | cancer de prostata grados
- Comment se procurer un certificat de signature électronique – certificat de signature électronique
- Tourist info valdaora: valdaora seekofelhütte
- Sql backend service: back end as a service deutsch
