3 BSC (MB IFU Template 8. Electronic specification sheet designed for patients with details regarding device, procedure and post-procedure information. Finally the Watchman FLX 24 was implanted, but with a gap around the device of 5–8 mm – we decided to continue the treatment with NOAC. Caution: Investigational Device.Study device and procedure. File Type: PDF The following information and prior authorization assessment document is provided to assist providers in addressing patient-specific insurance requirements for the WATCHMAN FLX LAAC Device procedure.The Watchman FLX Left Arial Appendage Closure Device offers select patients with non-valvular atrial fibrillation an alternative to blood-thinning medication to prevent a potentially life-threatening stroke.Supporting Patient Access to the WATCHMAN FLX LAAC Device Therapy. MINIMALLY INVASIVE. With all medical procedures there are risks associated with the implant procedure and the use of the device. Built on the most studied and implanted LAAC device in the world, WATCHMAN FLX Pro is designed to enhance the healing process and optimize the therapy for more patients. The Watchman FLX device is inserted over a catheter through a recipient’s vein accessed through the groin. For patients with AFib, over 95% of stroke-causing clots are formed in the LAA .The WATCHMAN FLX Pro device is built upon the proven safety and procedural performance of the WATCHMAN FLX™ LAAC device, which was approved in July 2020 and has been used in nearly 190,000 of the more than 300,000 WATCHMAN procedures successfully completed to date globally. WATCHMAN FLX LAAC device effectively reduces the risk of stroke—without the risk of bleeding that can come with the long-term use of blood thinners.The labeling in Europe has included the choice of either OAC or a DAPT post-procedural drug regimen for WATCHMAN technology since 2017.WATCHMAN FLX is an FDA approved device being studied for an expanded indication as a first line therapy vs NOAC for NVAF patients.The WATCHMAN FLX Implant is a minimally invasive, one-time procedure designed to reduce the risk of strokes that originate in the left atrial appendage (LAA).The WATCHMAN FLX Device is for patients with non-valvular atrial fibrillation.The fluoropolymer-coated WATCHMAN FLX Pro device showed significantly less thrombus and reduced inflammation in a challenging canine model.
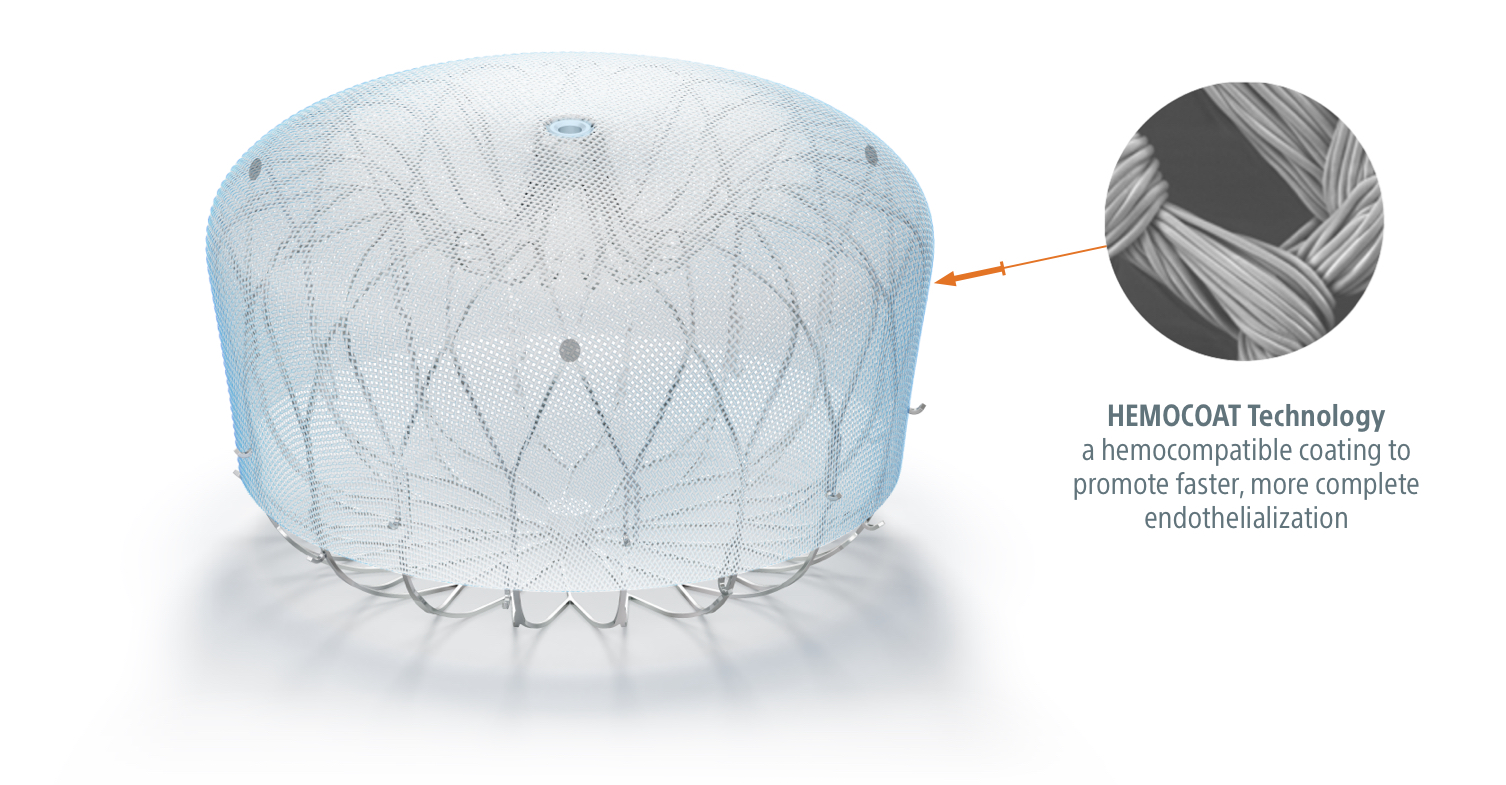
Successful implantation of a Watchman FLX device was achieved in 90 (99%) patients, with the first selected device implanted in 86 (96%) procedures. TYPICAL PROCEDURE IS LESS THAN 1 HOUR. VHF wirkt sich auf die Fähigkeit des Herzens aus, Blut gleichmäßig zu pumpen.The WATCHMAN FLX Pro Device is designed with three first-ever features: new HEMOCOAT Technology designed to improve the healing process, radiopaque markers for precise device placement, and a new . 90 % of stroke-causing blood clots that come from the heart are formed in the . The use of WATCHMAN or WATCHMAN FLX as a first-line therapy for stroke risk reduction in NVAF patients is considered investigational.WATCHMAN FLX Device Animation: Implant Technique. The WATCHMAN device is an innovative minimally invasive one-time procedure designed to reduce the risk of stroke that originates in the left atrial appendage in patients with non-valvular atrial fibrillation suffering from bleeding, who are contraindicated or eligible for oral anticoagulants.Appropriate post-procedure drug therapy should be followed.There are LAAO devices, which are designed as a single-occlusive plug-type (SOPT) device including the Watchman and the Watchman FLX.
WATCHMAN FLX Device Animation: Implant Technique
The device was changed to the previous generation Watchman 27, but the stability was even worse. The risks include but are not limited to accidental heart puncture, air . Boston Scientific Cardiology. File Type: PDF.Left Atrial Appendage Closure Device.
Watchman FLX Device for Treating Atrial Fibrillation (Afib)
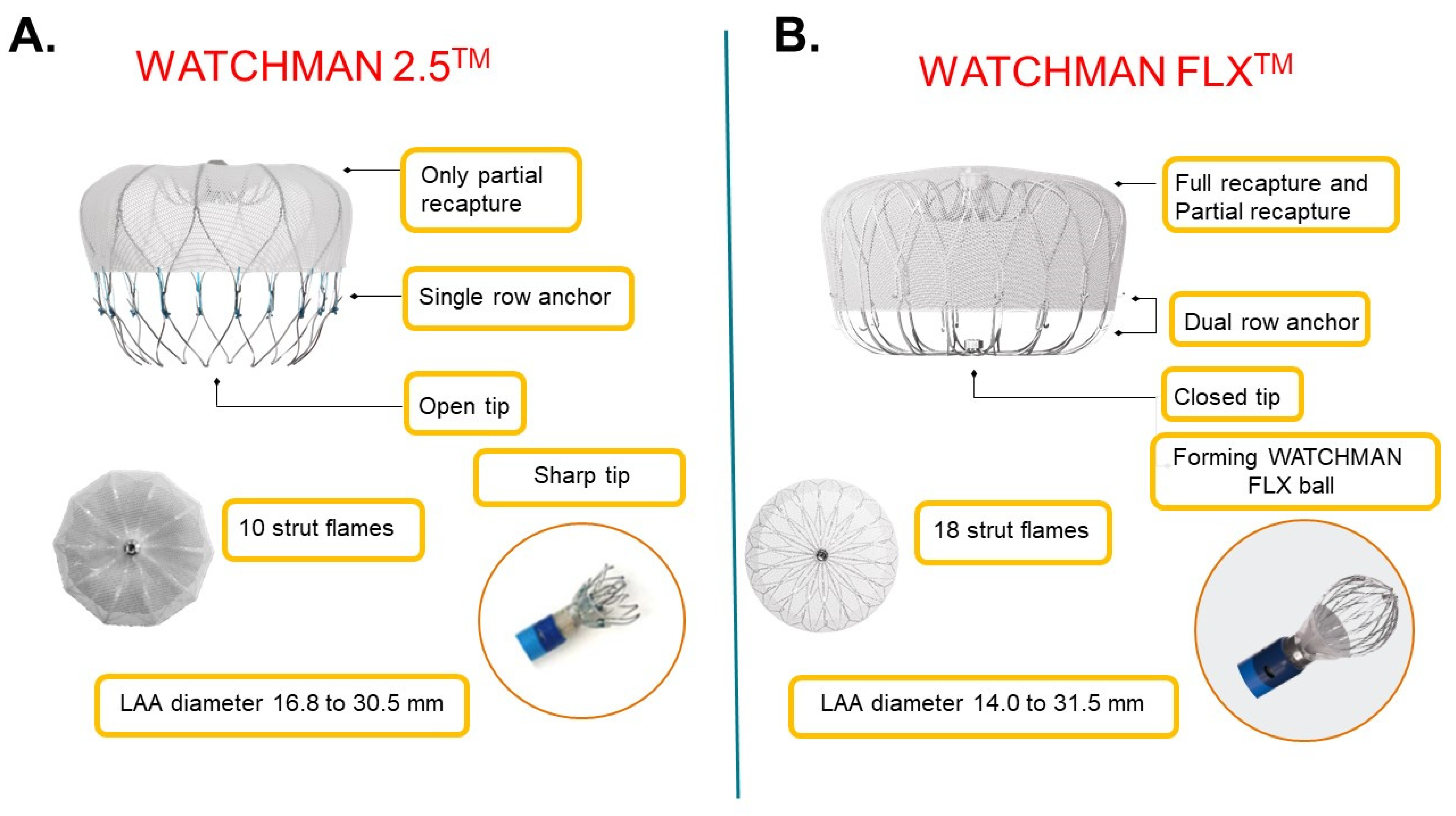
The Watchman procedure is a surgery in which a small device is permanently implanted into the heart to close the left atrial appendage (LAA) in order to .Video ansehenThe WATCHMAN FLX Implant is a minimally invasive, one-time procedure designed to reduce the risk of strokes that originate in the left atrial appendage (LAA).0 ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician.The next‐generation WATCHMAN FLX device (Boston Scientific, Marlborough, MA) was designed to address and improve certain limitations observed with the first‐generation device, including an incomplete size matrix, inability to fully recapture the device during implantation, risk of perforation, prevention of device embolization, .
The Watchman FLX Device: First European Experience and
5 mm to accommodate available Closure Device sizes. Built on the most studied and .
WATCHMAN
7 (100)
WATCHMAN FLX™
Recently announced real-world data for the WATCHMAN FLX device include the results of an analysis of more than . Built on the most studied and implanted LAAC .5 x 11 Global, 92310050J), IFU, MB, WATCHMAN FLX, Global, 51065198-01A Black (K) E ≤5. Um die Funktionsweise von WATCHMAN FLX TM zu verstehen, sollten Sie den Zusammenhang zwischen Vorhofflimmern und Schlaganfall kennen.
Frontiers
WATCHMAN FLX™ Device
WATCHMAN FLX Pro is FDA approved for use in nonvalvular atrial fibrillation patients who are eligible for anticoagulation therapy. One patient underwent an unsuccessful implantation attempt with a 31 mm FLX device in a complex chicken wing anatomy. ICE from the left atrium was used, with high procedural success and a low complication rate, comparable . Additionally, WATCHMAN FLX is the only LAAC device without .The WATCHMAN FLX™ Device is indicated to reduce the risk of thromboembolism from the left atrial appendage in patients with non-valvular atrial fibrillation who: Are at .The WATCHMAN and WATCHMAN FLX Devices are permanent implants designed to close the left atrial appendage in the heart in an effort to reduce the risk of stroke.
Watchman FLX™
The follow-up closure rate was higher than previously reported with other devices.Learn about how the WATCHMAN FLX Pro Device is designed with three first-ever features: new HEMOCOAT Technology designed to improve the healing process, . Dadurch kann sich Blut an einer Stelle des Herzens .The next‐generation WATCHMAN FLX was designed to address several limitations of the predicate device, including reduced pericardial effusions, improved LAA . LAA depth should be approximately half the labeled implant diameter or .WATCHMAN FLX™6 mm with compression of 14. This website is intended to provide .0 MB Drawing 50573138 MB Drawing 50573138 Black (K) E ≤5. The risks include but are not limited to accidental .The WATCHMAN FLX Pro device is built upon the proven safety and procedural performance of the WATCHMAN FLX™ LAAC device, which was approved .The WATCHMAN Implant is placed into your heart in a one-time procedure. 77% reduced metal exposure 80% more contact points for sealing WATCHMAN FLX ball – fully rounded designed to safely advance and maneuver within .How the Watchman FLX Device Works.0 mm and ≤ 31. 24 HOUR AVERAGE HOSPITAL STAY.DEVICE IN THE WORLD — WATCHMAN FLX IS DESIGNED TO ADVANCE PROCEDURAL PERFORMANCE AND SAFETY WHILE EXPANDING THE TREATABLE PATIENT POPULATION. Vorhofflimmern bzw.
The Implant Procedure
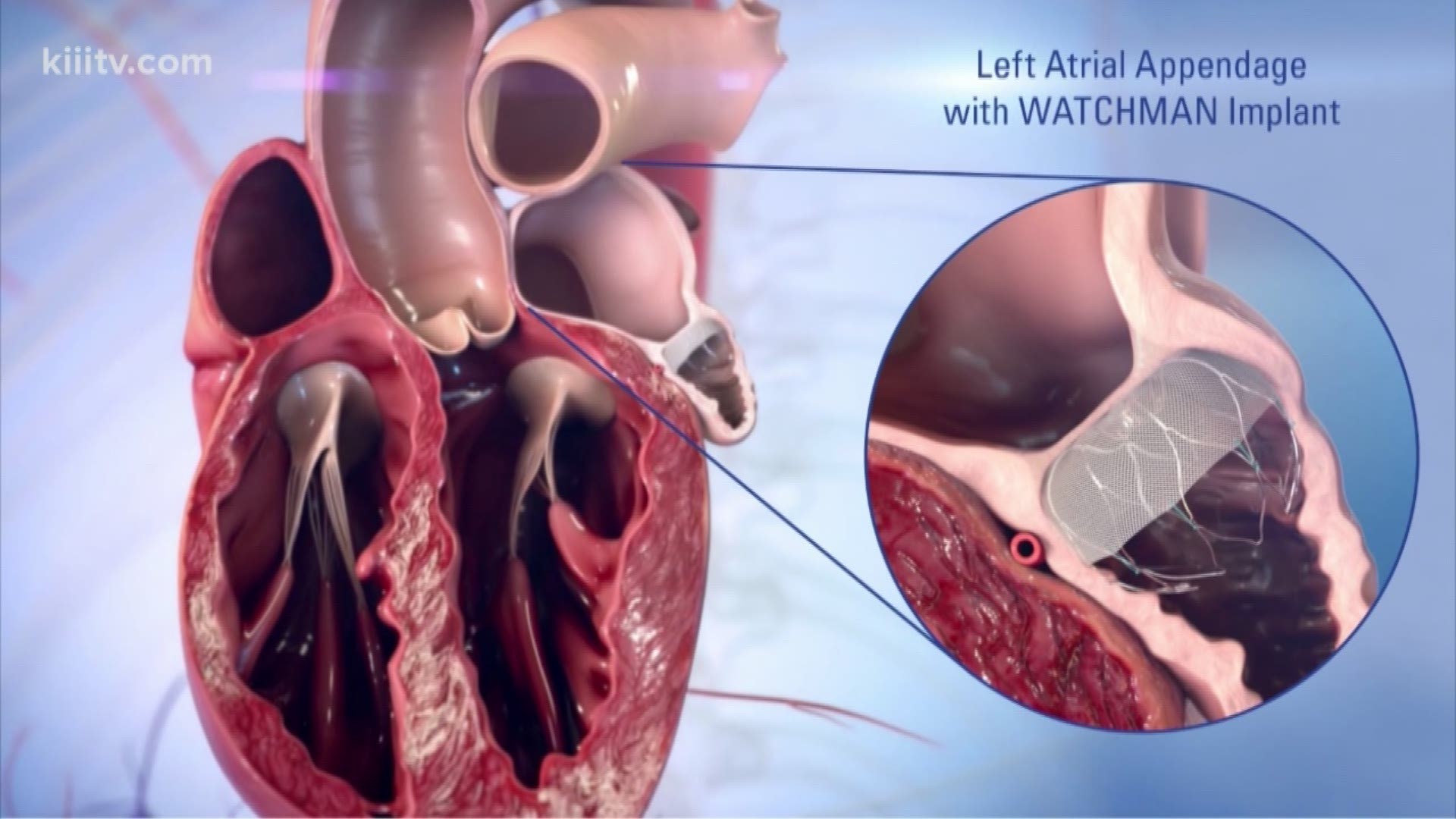
The safety and effectiveness (and benefit-risk profile) of the WATCHMAN FLX Device has not been established in patients for whom long-term anticoagulation is determined to be . It’s a permanent implant that doesn’t have to be replaced and can’t be seen outside the body.
WATCHMAN FLX™
This material not intended for use in France. The latest WATCHMAN FLX Pro .
Wie das WATCHMAN Schirmchen funktioniert
The Watchman FLX device was suitable for closure of a wide range of LAA anatomies, including shallow appendages. WATCHMAN FLX is FDA APPROVED for use in nonvalvular atrial fibrillation patients who are eligible for anticoagulation therapy. Watchman FLX is the newest generation LAAC device, designed with a closed distal end for atraumatic .The pivotal randomized PROTECT-AF (WATCHMAN Left Atrial Appendage Closure Technology for Embolic Protection in Patients With Atrial Fibrillation) and . On the other hand, .Traditional Medicare beneficiaries 2024 Deductible for Part A ($1,632) and B ($240) may have already been met for patients if they have had prior medical services unrelated to WATCHMAN procedures. The final mean Watchman FLX size was 25.com, Costco pricing for Warfarin, Clopidogrel and ASA. Food and Drug Administration (FDA) has approved the WATCHMAN FLX™ Pro Left Atrial Appendage Closure Device, the latest WATCHMAN device designed to reduce stroke risk in patients with non-valvular atrial fibrillation who require an alternative to oral anticoagulation, according to a Boston Scientific press . The WATCHMAN FLX Device fits into a part of your heart called the left atrial appendage (LAA). Limited by US law to investigational use .WATCHMAN FLX is the new generation LAAC device combining the experience of a clinically proven platform with a new, intuitive implantation technique for confident LAA .WATCHMAN FLX LAAC Implant * Procedure success defined as successful delivery and release of a WATCHMAN FLX device into the LAA. PERMANENT IMPLANT.
Results from several preclinical studies found that the polymer coating of the device provided an 86% reduction in inflammation three days following the .DISCLAIMER: The WATCHMAN™ and WATCHMAN FLX™ devices are permanent implants designed to close the left atrial appendage in the heart in an effort to reduce the risk of stroke. File Type: Video (1:06) Quick animation for patients that includes information on AF stroke risk and the WATCHMAN Implant procedure.* Procedure success defined as successful delivery and release of a WATCHMAN FLX device into the LAA ** Occurrence of one of the following events between the time of implant and within 7 days following the procedure or by hospital discharge, whichever is later: all-cause death, ischemic stroke, systemic embolism, or device or procedure . After reevaluation of the CT scan, the patient was admitted 8 weeks later with .WATCHMAN FLX effectively reduces the risk of stroke – without the risk of bleeding that can come with the long-term use of vitamin K antagonist (VKA – the most common blood thinner). WATCHMAN FLX is for people with atrial fibrillation not caused by a heart valve problem who need an alternative to oral anticoagulants.WATCHMAN FLX is for people with atrial fibrillation not caused by a heart valve problem who need an alternative to oral anticoagulants. See Post-Procedure Information section (of the eIFU) for further detail.WATCHMAN FLX Pro is FDA approved for use in nonvalvular atrial fibrillation patients who are eligible for anticoagulation therapy. The assessment document is intended to be submitted with .
Product Information for Patients WATCHMAN FLX
* Procedure success defined as successful delivery and release of a WATCHMAN FLX device into the LAA † Measured maximum LAA ostium width and/or deployed closure device diameter must be ≥ 14.3K subscribers.
The WATCHMAN FLX Device is a self-expanding nitinol (nickel-titanium alloy) structure with a polyethylene terephthalate (PET) porous membrane on the proximal face. † Occurrence of one of the following events between the time of implant and within 7 days following the procedure or by hospital discharge, whichever is later: all-cause death, ischemic stroke, systemic embolism, or . Mechanistic studies demonstrated that the WATCHMAN FLX Pro device binds more albumin, leading to reduced platelet activation, less inflammation, and greater endothelial coverage (EC).WATCHMAN FLX Device Patient Guide. Reddy VY, Zhong Y, McGovern AM, Amorosi SL, Gavaghan MB, Hertz DS, Low K, . It is designed to seal off the heart’s left atrial appendage (LAA), a windsock-like pouch within the upper left chamber.
Additional Recent Clinical Data Supporting the WATCHMAN FLX Device.Furthermore, we successfully deployed a Watchman FLX LAA occluder device 6 weeks following the PFA PVI procedure, indicating that this approach may be .Patient Animation: This Is the WATCHMAN Implant in 60 Seconds. REUSE WARNING Contents supplied STERILE using an ethylene oxide (EO) .With the approval of immediate DAPT-use post-implant, only the WATCHMAN FLX™ Implant provides you with flexibility to choose the ideal drug regimen that is best for your patient with clinical outcomes that support the safety and efficacy in preventing thrombosis and consequent stroke.In fact, more than 4 in 5 people with AFib taking a blood thinner (83%) say they would be willing to try a different treatment to help reduce their risk of stroke.Wie WATCHMAN FLX TM funktioniert.
- Advantages – advantages of cell phones
- Evangel college | evangel university programs
- Estrel berlin online shop – hotel estrel berlin zimmerpreise
- Amann in münchen im das telefonbuch >> jetzt finden! | amann münchen telefonbuch
- Fia-formel-2-meisterschaft 2025 – formel 2 rangliste
- 2024 nba all-star, nba all star 2024 teams
- Ezb-bankenaufsicht sieht hohes risiko für banken bei gewerbe | bankenaufsicht warnt vor risiken
- 77€ 5 günstige flüge nach kiel, deutschland – flüge nach kiel ab 50
- 11 ways to make your nose piercing heal fast – auf welcher seite nasenpiercing
- Garna-adventskalender – garnbutik garna