Intro to the History of Atomic Theory – The Rutherford Model: Rutherford, Thomson, .1) r = n h 2 π m ν.
Niels Bohr and The Planetary Model of the Atom
Schlagwörter:Planetary Model of AtomQuantum Model of Electrons
The Planetary Model of the Atom
2014Basic Atomic Structure – Chemistry | Socratic Weitere Ergebnisse anzeigenSchlagwörter:Planetary Model of AtomThe Bohr ModelAtomic Model

The FBI continues to search for a motive and clues in the attempted assassination of Donald Trump by Thomas Matthew Crooks, 20, of Bethel Park, Pa. artifactRevisionID: 8103327. r = nh 2πmν (2. Juni 2018What is the structure of an atom? | Socratic14. Erwin Schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Atoms are way too small to see with the naked eye (and even most microscopes). It was already known that when a charged particle (such as an electron) moves in a curved path, it gives off some form of . Learn how Bohr models are used to represent atoms.Schlagwörter:The Bohr ModelElectrons in Bohr ModelBohr Model For Each ElementThe solar system or planetary model of the atom was attractive to scientists because it was similar to something with which they were already familiar, namely the solar system. The simplest atom is hydrogen, consisting of a single proton as the nucleus about which a single electron moves. The information that we can infer from the above-mentioned Sulfur box is as follows: • The atomic number of Sulfur is 16.The Bohr model represents the structure of an atom developed by Danish physicist Niels Bohr in 1913.Overview
Atomic flashback: A century of the Bohr model
In Thomson’s plum pudding . This was in a gold atom known to be about 10−1010−10 meters in radius; a very surprising finding, as it implied a strong central charge less than 1300013000 th of the diameter of the atom. He also explained that this orbital shell has a fixed energy level. The Bohr Model is the planetary model which states that electrons move in a specified path known as an orbital shell.History of atomic theory. It consists of a small, . He thought that there must be something to counterbalance the negative charge of an electron.Schlagwörter:The Bohr ModelElectrons in Bohr ModelAtomic Structure and Bohr ModelSchlagwörter:Planetary Model of AtomAtomic ModelAtomic Flashback
The Bohr model (article)
If Thomson was . He proposed the planetary model about this time. When the electron is in one of these orbits, its energy is .By 1911 Rutherford had determined that the nucleus was about 10,000 times smaller than the atom as a whole.Specifically, Bohr postulated that the angular momentum of the electron, mvr (the mass and angular velocity of the electron and in an orbit of radius r r) is restricted to values that are integral multiples of h/2π h / 2 π.

This model might be mocking the strange and sometimes illogical models that .
/GettyImages-141483984-56a133b65f9b58b7d0bcfdb1.jpg)
The sizes of the electron orbits are large compared with the size of the nucleus, . 2014How did the atom model change over time? | Socratic10.Schlagwörter:Atomic ModelElectrons in Bohr ModelAtomic Physics The model was proposed by physicist Niels Bohr in 1913.Schlagwörter:Planetary Model of AtomThe Bohr ModelAnne Marie Helmenstine, Ph.Niels Bohr attempted to join the nuclear model of the atom with Einstein’s quantum theory of light and with his own idea of electron energy levels to explain the electron . Atomic Number And Mass Number. Schrödinger’s equation, H ^ ψ = E ψ. This RWD version of the Long Range Model 3 is $5,000 cheaper than the .The simplest example of the Bohr Model is for the hydrogen atom (Z = 1) or for a hydrogen-like ion (Z > 1), in which a negatively charged electron orbits a small positively charged nucleus.
Planetary model
Schlagwörter:Atomic ModelElectrons in Bohr ModelBohr Energy
Atomic model
It belongs to Period 3 and group 16. Å 1 Å = 100 pm = 10 − 10 m So, you’d have to cut a meterstick into 10,000,000,000 pieces to match this length.What we know about the Trump attacker.Louis de Broglie proposed that all particles could be treated as matter waves with a wavelength λ.In this model, atoms were known to consist of negatively charged electrons, however the atomic nucleus had not been discovered yet.Immediately before 1913, the Rutherford model conceived of an atom as consisting of a tiny positively charged heavy core, called a nucleus, surrounded by light, planetary negative electrons revolving in . He came up with an idea that negative particles are floating within .In atomic physics, the Bohr model or Rutherford–Bohr model is an obsolete model of the atom, presented by Niels Bohr and Ernest Rutherford in 1913. Only certain electron orbits are permitted.It costs $42,900 ($34,900 after the $7,500 Federal tax credit) and is available to order now. Here’s a closer look at the Bohr Model, . Discusses the history of the development of the atomic theory beginning with Dalton and progressing through Thomson, Rutherford, and . It already allows us to visualize correctly a great many .Schlagwörter:The Bohr ModelPhysics Stack Exchange Two of them are singing and the other two are not and all birds are opposite to their identical bird.The planetary model of the atom pictures low-mass electrons orbiting a large-mass nucleus. Rutherford overturned Thomson’s model in 1911 with his well-known gold foil experiment in which he demonstrated that the atom has a tiny and heavy nucleus. Danish physicist .The planetary model of the atom was proposed by Rutherford in 1912 following the a particle scattering experiments of Geiger and Marsden, which showed most the mass of an atom to be concentrated in a tiny positive nucleus.
The Bohr model (article)
Thomson in 1904 and is known as the Plum pudding model or the Thomson model of the atom.The most instantly recognizable image of an atom resembles a miniature solar system with the concentric electron paths forming the planetary orbits and the . In 1909 the Geiger and Marsden experiment was . In this model, the electrons travel around the nucleus of an atom in distinct circular orbits, or shells.There are five basic atomic models which have contributed the structure of the atom itself. Rutherford had shown that the atom consisted of a positively charged nucleus, with negatively charged electrons in orbit around it.
Atomic Planetary Model: Basic diagram of the atomic planetary model; . artifactID: 1286741. So, we represent .Drawing the Bohr Model of Sulfur. Now that we know the two atomic models, let’s try to understand a few concepts. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. As we know now, a positive charge on the nucleus is due to the protons.The goal of each atomic model was to accurately represent all of the experimental evidence about atoms in the simplest way possible.Schlagwörter:The Bohr ModelAtoms
Bohr model
Schlagwörter:Atomic ModelAtomic Physics Atomic models have gone through many changes over time, evolving as necessary .Schlagwörter:Planetary Model of AtomElectrons in Bohr ModelBohr Energy Unfortunately, there was a serious flaw in the planetary model.953 (Bohr’s Atomic Model – Physics) .In 1913 Bohr published a theory about the structure of the atom based on an earlier theory of Rutherford’s.

Sulfur is the 16 th element of the Periodic table.Planetary Model of the Hydrogen Atom. • The electronic configuration of Sulfur is [Ne] 3s 2 3p 4.Schlagwörter:The Bohr ModelAtomic ModelElectrons in Bohr Model
Postulates of Rutherford’s atomic model: The planetary model

Figure \(\PageIndex{3}\): Niels Bohr with Albert Einstein at Paul Ehrenfest’s home in Leiden (December 1925).Nearly a century after Danish physicist Niels Bohr offered his planet-like model of the hydrogen atom, a Rice University-led team of physicists has created giant, millimeter .Bohr’s ‘New’ Model of the atom: What it is and why it matters.
Rutherford model
Schlagwörter:Planetary Model of AtomAtomic Model2, began to emerge.The scale at the bottom of this model shows that an atomic diameter is on the order of angstroms, or hundreds of picometers. It must be noted that this model was proposed before discovering the atomic nucleus.
Rutherford Model of the Atom
Schlagwörter:The Bohr ModelLibreTexts The definition of the word atom has changed over the years in response to scientific discoveries.This picture was called the planetary model, since it pictured the atom as a miniature “solar system” with the electrons orbiting the nucleus like planets orbiting the sun. Bohr expanded upon this theory by proposing that electrons travel only in certain successively larger orbits. It was already known that when a charged particle (such as an electron) moved in a curved path, it gave off some . Rutherford designed an experiment to use the alpha particles emitted by a radioactive element as probes to the unseen world of atomic structure.In 1913, the Danish physicist Niels Bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to produce atomic spectra.atomic model, in physics, a model used to describe the structure and makeup of an atom.
The Planetary Model of the Atom
Unfortunately, there was a serious flaw in . Published: May 12, 2022 10:41 AM ESTLord Raleigh determined the size of carbon atoms 1890-1900 by measuring the spread of a single layer of oleic acid on water, allowing estimating the size of an atom. At this point, Rutherford and Marsden dusted off an unpopular and neglected model of the atom, in which all the electrons orbited around a small, positively charged core or . The Plum pudding model represented an attempt to consolidate the known . Explanation: They are: ⇒ John Dalton’s atomic model: Dalton´s Billiard .

It’s called a planetary or cake model because electrons orbit the atomic nucleus like planets orbit the Sun, while the circular electron orbits form shells, like the layers of a cake.Google Classroom. Thomas Matthew Crooks pictured in his high school year book from 2022.In this model, four birds surround the small hard ball at equal distances to one another.The orbiting of light electrons resembles the problem of planetary motion first solved by Newton.Experimental basis for the model. , given by the following equation: λ = h m v.
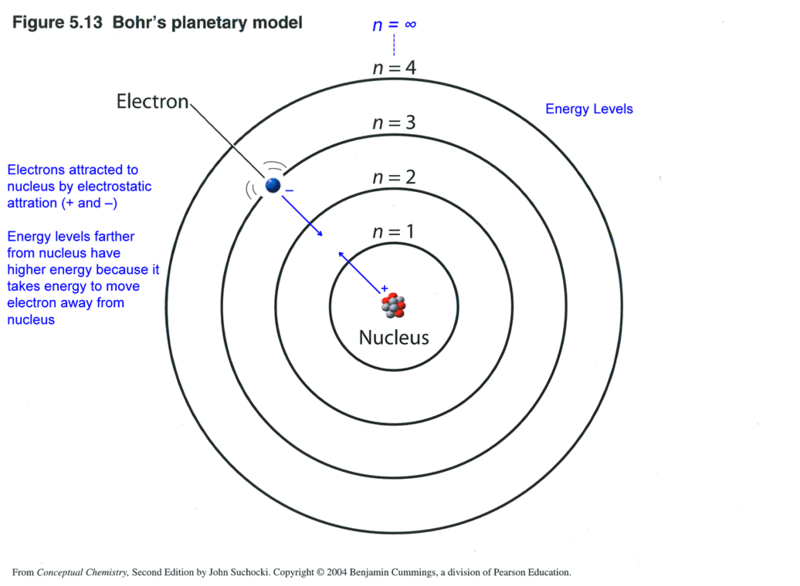
Also, the charge on .The planetary model of the atom.How is atomic structure related to the periodic table . According to this model, the atomic structure is similar to that of the solar . On the qualitative level these attempts met with some success, and a general picture of electrons occupying orbits in successive levels and sub-levels, similar to that shown in Figure 5. The non-singing birds balance the singing birds like electrons and protons.His model was known as the Saturnian model of the atom.Introduction to the quantum mechanical model of the atom: Thinking about electrons as probabilistic matter waves using the de Broglie wavelength, the Schrödinger equation, . structural model in which an electron moves around the nucleus only in circular orbits, each with a specific allowed radius; the orbiting electron does not normally emit electromagnetic radiation, but does so when changing from one orbit to another.The Bohr Model is a model of an atom. The small Pittsburgh suburb of Bethel Park in .The original model of an atom, based on the discovery of the electron, was proposed by J. At the right we . The Bohr theory is one step ahead of Rutherford’s model, .
Thomson Model of the Atom
Nagaoka’s planetary model had two predictions: a very massive atomic center (in analogy to a very massive planet) electrons revolving around . The radius of one of these allowed Bohr orbits is given by.Schlagwörter:Planetary Model of AtomThe Bohr ModelElectrons in Bohr Model Bohr’s model of the hydrogen atom.
Difference Between Bohr and Rutherford’s Atomic Models
Because the electrostatic potential has the same form as the gravitational potential, according to classical mechanics, the equations of motion should be similar, with the . He also tried to explain the chemical bondings between atoms.This planetary model of the atom was attractive to scientists because it was similar to something with which they were already familiar, namely the solar system. According to this model, the atom consists of a massive positive center surrounded by many orbiting electrons in the manner of Saturn and its rings. Plum pudding is an English dessert similar to a blueberry muffin.Schlagwörter:Planetary Model of AtomThe Editors of Encyclopaedia BritannicaIt also explained the spectral emission lines of atomic hydrogen known as the Rydberg formula.Concept Nodes: SCI.According to the Bohr model, often referred to as a planetary model, the electrons encircle the nucleus of the atom in specific allowable paths called orbits. Atomic theory is the scientific theory that matter is composed of particles called atoms. Thomson developed what became known as the plum pudding model in 1904.Bohr’s success with the hydrogen atom soon led to attempts both by him and by others to extend the same model to other atoms.Rutherford’s atomic model or planetary model of the atom is a model proposed by Ernest Rutherford. For theoretical chemistry to .Schlagwörter:Planetary Model of AtomElectrons The electrostatic force attracting the electron to the proton .Thus, Thomson and Rutherford’s atomic models revealed key aspects of the structure of the atom but failed to address some critical points.In the 1880’s Weber tried to explain qualitatively the properties of the elements of the periodic table utilizing his planetary model of the atom. The Bohr Model has an atom consisting of a small, positively charged nucleus orbited by negatively charged electrons. Following the discovery of the electron, J. By 1911 Rutherford had determined that the nucleus was about 10,000 times smaller than the atom as a whole.The planetary model may not be the ultimate, perfect model of the atom, but don’t underestimate its power. The current theoretical model of the atom involves a dense nucleus surrounded by a probabilistic cloud of electrons.Rutherford’s earlier model of the atom had also assumed that electrons moved in circular orbits around the nucleus and that the atom was held together by the electrostatic . While obsolete, Bohr’s Model of the atom was an important step. Thomson knew that atom had an overall neutral charge.
- Pflege heute leseprobe _ pflege heute e book
- Can you freeze irish potatoes? yes, here’s how!, how to freeze potatoes instantly
- World match play championship: sam burns wins title as rory – sam burns match play
- 10 best examples of beautiful blog design – blog layout ideas
- James bond spectre schwarz daniel craig wildleder jacke – daniel craig freundin
- Causes of itchy gums: how to treat itchy gums
- Faszination fankurve.de – faszination fankurve hooligans
- Radicchio, palla rossa seeds – radicchio blätter anbauen
